AbstractPurposePatients with advanced biliary tract cancer (BTC) have a poor survival. We aim to evaluate the efficacy and safety of nab-paclitaxel plus gemcitabine and cisplatin regimen in Chinese advanced BTC patients.
Materials and MethodsEligible patients with locally advanced or metastatic BTC administrated intravenous 100 mg/m2 nab-paclitaxel, 800 mg/m2 gemcitabine, and 25 mg/m2 cisplatin every 3 weeks. The primary endpoint was progression-free survival (PFS). The secondary endpoints included overall survival (OS) and adverse events, while exploratory endpoint was the association of biomarkers with efficacy.
ResultsAfter the median follow-up of 25.0 months, the median PFS and OS of 34 enrolled patients were 7.1 months (95% confidence interval [CI], 5.4 to 13.7) and 16.4 months (95% CI, 10.9 to 23.6), respectively. The most common treatment-related adverse events at ≥ 3 grade were neutropenia (26.5%) and leukopenia (26.5%). Survival analyses demonstrated that carcinoembryonic antigen (CEA) levels could monitor patients’ survival outcomes. A significant increase in the number of infiltrating CD4+ cells (p=0.008) and a decrease in programmed death-1–positive (PD-1+) cells (p=0.032) were observed in the response patients.
IntroductionBiliary tract cancer (BTC) is a diverse malignant disease entity arising from bile duct epithelial cells, classified into intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma, and gallbladder carcinoma [1,2]. The incidence of BTC is increasing globally [1,3]. The majority of patients diagnosed at unresectable locally advanced or metastatic disease with 5-year overall survival (OS) rate approximately 5% [4,5]. Additionally, recurrence occurred in more than half of surgical patients [6,7]. Therefore, systemic chemotherapy plays an important role in the treatment of advanced BTC.
Based on the results of ABC-02 trial, gemcitabine and cisplatin have been defined as first-line standard-of-care in patients with advanced BTC for more than 10 years [5]. Unfortunately, even with this potent doublet chemotherapy, the median OS remains less than 1 year [8,9]. In recent years, a triple chemotherapy regimen including nab-paclitaxel, gemcitabine, and cisplatin showed promising results in a phase II study with a median progression-free survival (PFS) of 11.8 months, a median OS of 19.2 months, and an objective response rate (ORR) of 45% [10]. A randomized phase III clinical trial further comparing this triple chemotherapy regimen with gemcitabine and cisplatin is currently underway (NCT03768414).
However, real-world outcomes from Jung et al. [11] showed that this triple chemotherapy regimen did not improve PFS and OS in patients with advanced BTC compared to standard chemotherapy. Additionally, the incidence of BTC has been reported to be relatively high in Asia [12,13], but the previous phase II study included patients only from the United States, the differences and consequences arising from ethnicity remain unexplored [10]. We, therefore, performed a phase II trial to evaluate the efficacy and safety of triple chemotherapy with nab-paclitaxel, gemcitabine, and cisplatin in Chinese patients with advanced BTC.
Materials and Methods1. Study oversightThis open-label, single-arm, phase 2 clinical trial was performed in accordance with the Declaration of Helsinki and was registered at Chinese Clinical Trial Registry (ChiCTR2000036850). This prospective study was conducted at West China Hospital of Sichuan University, and patients were required to provide written informed consent prior to entering the study. All authors attested that the study was conducted in accordance with the protocol and all its amendments. And, all authors had access to the study data and reviewed and approved the final manuscript.
2. PatientsThe eligibility criteria for patients enrolled in this study were as follows: ≥ 18 years of age; histologically confirmed BTC; diagnostic imaging documented metastatic, recurrent or locally advanced unresectable disease; Eastern Cooperative Oncology Group (ECOG) performance status (PS) score ≤ 2; adequate bone marrow, renal, hepatic, and coagulation functions. The exclusion criteria were: prior lines of systemic chemotherapy (gemcitabine-containing double chemotherapy without progression was permitted); pregnancy or lactating woman; serious medical disease or psychiatric complications potentially affecting research participation; active infections; prior hematological or solid malignancies in the past 5 years (except basal cell skin cancer and carcinoma in situ of the cervix); uncontrolled peripheral or central nervous system metastasis lesion; and not eligible for enrollment in the judgment of the investigator.
3. TreatmentsEnrolled patients received sequential intravenous 100 mg/m2 nab-paclitaxel (Hengrui, Lianyungang, China), 800 mg/m2 gemcitabine (Hansoh, Lianyungang, China), and 25 mg/m2 cisplatin (Hansoh) (30-, 30-, and 60-minute infusions, respectively) on days 1 and 8 every 3 weeks. Enrolled patients were scheduled to administrate 6-8 cycles of treatment. For uncontrolled grade 3-4 adverse events (AEs), dose interruption was allowed and dose level adjustments were restarted with a 30% dose reduction when the patients recovered to ≤ 2 grade. A delay of less than 2 weeks was allowed for the next cycle of chemotherapy.
4. EvaluationSafety was monitored throughout the study and AEs were graded by the investigator according to National Cancer Institute Common Terminology Criteria for Adverse Events ver. 5.0. The tumor response was assessed based on the criteria of Response Evaluation Criteria in Solid Tumor ver. 1.1. All treatment evaluation was assessed both by the investigator and by independent central review.
5. Study endpointsThe primary endpoint was PFS. The secondary endpoints included OS, ORR, disease control rate (DCR), AEs, levels of tumor markers including carbohydrate antigen (CA) 19-9 and carcinoembryonic antigen (CEA), and lymphocyte infiltration in the tumor microenvironment (TME). PFS was defined as the time from the start of the first dose of treatment to documented disease progression or death from any cause, whichever came first. OS was defined as the time from enrollment to death or when the patient was censored at last contact.
6. Multiplex immunofluorescence stainingMultiplex immunofluorescence staining was performed in 20 samples from five complete response (CR)/partial response (PR) patients (response patients) and five progression disease patients (non-response patients). The as-prepared tumor sections were stained according to the instructions of six-color multiplex fluorescence immunohistochemical staining kit (catalog No. abs50015, Absin, Shanghai, China). The antibodies involved in experiment include CD4 (diluted at 1:300, #48274, Cell Signaling Technology), Foxp3 (diluted at 1:200, #98377S, Cell Signaling Technology), CD8 (diluted at 1:500, #85336, Cell Signaling Technology), programmed death-1–positive (PD-1+; diluted at 1:500, #86163, Cell Signaling Technology) and TIGIT (diluted at 1:200, ab243903, Abcam, Cambridge, MA). The nuclei were stained with DAPI before sealing, and all sections were scanned by a fluorescent scanning camera (Vectra3, AKOYA Bioscience, Marlborough, MA).
7. Statistical analysisThe efficacy and safety population included all participants who met the eligible criteria and who received at least one complete treatment cycle. Quantitative variables were described using the median and range. Qualitative variables were described using frequency, rates, and the 95% confidence interval (CI). Survival endpoints were depicted by the Kaplan-Meier curve with hazard rate (HR) and its 95% CI, and log-rank test was used to assess the difference in survival outcomes by subgroups. The “surv cutpoint” function in the “survminer” package of the R software was used to determine the best cutoff value for tumor markers. The HR in subgroup analysis was calculated by the Cox proportional-hazards regression. Statistical differences were calculated by GraphPad Prism software (ver. 9, GraphPad Software Inc., San Diego, CA) using unpaired student’s t test. All statistical tests used a 2-sided significance level of 5%. The statistical analyses were performed using PASW Statistics ver. 25 (SPSS Inc., Chicago, IL) and the R software ver. 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results1. Patient characteristicsFrom May 2019 to September 2021, 37 patients were enrolled and 34 patients received at least 1 cycle of the nabpaclitaxel, gemcitabine, and cisplatin treatment (Fig. 1). The median age was 57 years (range, 41 to 70 years), and 18 of 34 patients (52.9%) were male. The majority of patients had ECOG PS=0 (70.6%), body mass index (BMI) < 23 (61.8%), radical resection (73.6%), and confirmed adenocarcinoma (88.2%). The most common tumor type was ICC (44.1%), and 32 of 34 patients (94.1%) had metastatic disease. The most common site of tumor metastasis was retroperitoneal lymph node (58.8%), followed by liver (35.2%) and peritoneum (14.7%) (Table 1). The median values of tumor markers CA 19-9 and CEA were 137.06 U/mL (range, > 2 to < 1,000) and 35.19 ng/mL (range, 0.76 to 612), respectively.
2. Treatment exposureOut of whole 34 patients, 31 (91.2%) were initially treated with the planed nab-paclitaxel plus gemcitabine and cisplatin regimen and three others were initially treated with the gemcitabine plus cisplatin, gemcitabine plus nab-paclitaxel or gemcitabine plus oxaliplatin, respectively, and they switched to the triplet chemotherapy regimen after participating in this study (S1 Table). Twenty-eight patients (82.4%) discontinued treatment, reasons of which were disease progression (n=17), completion of planned treatment (n=6), intolerable AEs (n=3), and patients’ decision (n=2). Further, more than half of patients received ≥ 6 cycles of triple therapy and 10 patients (29.4%) experienced dose reduction (S1 Table). These patients received a median of 6 treatment cycles (range, 1 to 11), and the median duration of treatment was 5.0 months (95% CI, 3.8 to 6.6).
3. EfficacyUpdated to February 28, 2023, the median duration of follow-up was 25.0 months (S2 Fig.), and 30 patients (88.2%) experienced disease progression or death. The median PFS was 7.1 months (95% CI, 5.4 to 13.7) (Fig. 2A). Subgroup analyses indicated that patients with BMI ≥ 23 kg/m2 had favorable PFS than those with lower BMI (15.5 vs. 6.1 months; HR, 0.34; 95% CI, 0.14 to 0.83; p=0.014). Besides, patients with squamous or adenosquamous carcinoma (7.9 vs. 3.9 months; HR, 3.42; 95% CI, 1.11 to 10.51; p=0.022) or with peritoneal metastasis (7.6 vs. 4.6 months; HR, 3.08; 95% CI, 1.11 to 8.51; p=0.023) (S3 Fig.) had significantly shorter PFS. The median OS was 16.4 months (95% CI, 10.9 to 23.6) (Fig. 2B). Subgroup analysis demonstrated in patients with BMI ≥ 23 kg/m2 had favorable OS (21.1 vs. 11.8 months; HR, 0.37; 95% CI, 0.14 to 0.96; p=0.035) (S4 Fig.). While, tumor location, disease status and metastatic number were not significantly associated with PFS nor OS.
Treatment response data were available for 34 patients (S5 Table). Two patients achieved CR (5.9%), nine patients achieved PR (26.5%), thus the ORR was 32.4% and DCR was 85.3%. Furthermore, five patients were rechallenged with the same triple regimen when disease progression or relapse after first-line treatment, resulting PR in one patient and stable disease in two patients, with a DCR of 60.0% (Fig. 2C-E).
4. SafetyFor the whole cohort, grade 3 or higher AEs occurred in 21 patients (61.8%). Neutropenia (26.5%) and leukopenia (26.5%) were the most common grade 3 or higher AEs, followed by thrombocytopenia (23.5%) (Table 2). In addition, among the five patients who rechallenged the triplet chemotherapy regimen, the most common AEs remained neutropenia and leukopenia.
5. Exploratory analysisBaseline and post-treatment tumor markers were available for all 34 patients and classified into high or low groups according to the optimal cutoff values (S6 Fig.). Survival analyses showed that the CEA levels and their post-treatment changes could predict patients’ survival outcomes. Whereas post-treatment CA19-9 levels were predictive of OS (Fig. 3). We performed multiplex immunofluorescence staining to detect immune biomarkers in pretreatment tumor biopsies obtained from 10 patients. A significant increase in the number of infiltrating CD4+ cells (p=0.008) and a decrease in PD-1+ cells (p=0.032) were observed in the response patients. There was no significant difference in the number of infiltrating CD8+, FoxP3+, or TIGIT+ cells in the response patients and non-response patients (Fig. 4).
DiscussionTo our knowledge, this was the first prospective phase II study accessing the efficacy of the triple chemotherapy including nab-paclitaxel plus gemcitabine and cisplatin in Asian patients with advanced BTC. For the whole 34 patients, the median PFS was 7.1 months, median OS was 16.4 months and ORR was 32.4%. These PFS and ORR outcomes were similar to those observed in a phase II gemcitabine plus nab-paclitaxel trial (median PFS of 7.7 months and ORR of 30.0%) [14], and in a phase III ABC-02 trial (median PFS of 8.0 months and ORR of 26.1%) [5]. While, after a median follow-up time of 25 months, our results indicated favorable OS than those double chemotherapy regimens (median OS of 11.7 months and 13.7 months, respectively) [5,14]. Additionally, we observed good symptomatic relief, especially abdominal pain, in patients who received the triple regimen. Therefore, when patients experienced disease recurrence, five of whom chose to re-challenge the triple regimen. Intriguingly, the ORR and DCR was still up to 20% and 60%, respectively. This may be one of the reasons influencing the results of our study.
Notably, the previous phase II study conducted in the United States reported significantly better efficacy in the triple regimen (median PFS of 11.8 months, median OS of 19.2 months, and ORR of 45.1%) than that of our study [10]. This discrepancy may be due to the differences in baseline characteristics of the participants. Our study included higher proportion of patients with metastatic disease (94.1% vs. 78.0%), lower proportion of ICC patients (44.1% vs. 63.0%), and higher level of CA19-9 (137 vs. 99 U/mL). As disease stage and CA19-9 levels were well-documented prognostic factors [15] and a collective study with individual data from ABC trials showed better OS from ICC patients [16], the differences in these potential prognostic factors may have contributed to differences in outcomes.
In the present study, patients were treated with reduceddose regime, which was recommended in the previous phase II trial. Given that only 28 of 60 patients (46.7%) in the previous study received the reduced-dose regimen and resulted lower ORR than that in the high-dose group (39.1% vs. 50.0%) [10], thus a reduced-dose may be associated with poor outcomes in the triple chemotherapy of this study. In addition, due to the influence of coronavirus disease 2019 (COVID-19), the chemotherapy cycles were forcibly prolonged, which may be one of the reasons that the survival outcomes in this study were inferior to that reported previously.
Notably, our study found that patients with BMI ≥ 23 kg/m2 had longer PFS (15.5 vs. 6.1 months) and OS (21.1 vs. 11.8 months) than those with lower BMI, similar to previously reported findings [17]. One potential explanation was that patients with higher BMI tended to have more fat which was positively correlated with survival outcome [18]. Another possibility was that people with more muscle mass may be included in the high BMI group, and muscle mass was also strongly associated with patient prognosis [19]. In addition, studies also showed that sarcopenia was associated with poor prognosis in cancer patients [20,21]. In the present study, 61.8% of the patients had a BMI < 23 kg/m2. This may be one of the reasons we did not achieve superior efficacy.
The triple chemotherapy regimen of nab-paclitaxel plus gemcitabine-cisplatin showed an acceptable safety profile among patients with BTC, and no unexpected toxicities were observed in the present study. The safety data in our study appear favorable compared with those of historical gemcitabine plus cisplatin [5] and gemcitabine plus nab-paclitaxel [14], and similar to those observed in the study of gemcitabine-cisplatin plus nab-paclitaxel triplet chemotherapy [10,11]. However, it should be noted that all safety data on triple regimens were from small-scale clinical studies, and large-scale clinical studies are still needed to validate the safety of triple chemotherapy.
Although previous studies have attempted to identify prognostic factors in advanced BTC, no reliable prognostic factors have been established currently [22]. Some studies reported that CA19-9 was as an independent prognostic factor in BTC [15,23]. However, in our study, we found that baseline CA19-9 was not independent prognostic marker. Consistent with previous studies [15,24], our results demonstrated that baseline CEA levels and their post-treatment changes could predict patients’ outcomes. Additionally, the characteristics of the TME have therapeutic significance [25,26]. In this study, we explored for the first time the differences of TME in patients with or without response to the triple chemotherapy. We found that the number of infiltrated CD4+ cells increased significantly (p=0.008) and the number of PD-1+ cells decreased significantly (p=0.032) in the responding patients. These potential prognostic factors may provide us additional basis for the treatment and prognostic monitoring of advanced biliary tract tumors. Further validation is still needed.
The present study had limitations. One limitation is that our study was a single-arm phase II clinical trial, which might have led to biased results. Further randomized controlled clinical trials are needed to confirm these findings. In addition, our study was conducted during the COVID-19 epidemic, and outbreak control with disruption of patient admissions was unavoidable.
In conclusion, this was the first prospective clinical trial to administrate the triple regimen of nab-paclitaxel plus gemcitabine and cisplatin in an Asian population. The use of this triple regimen in patients with advanced BTC resulted favorable OS better than the current standard double chemotherapy regimen. Moreover, potential prognostic factors of CEA levels, number of CD4+ cells and PD-1+ cells may help us maximize the efficacy benefit.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the China Ethics Committee of Registering Clinical Trails (ChiECRCT20200193). Informed consent was obtained from all individual participants included in the study. Author Contributions Conceived and designed the analysis: Liu T, Li Q, Zeng S, Zhu Q, Gou H. Collected the data: Liu T, Li Q, Lin Z, Liu C, Pu W, Zeng S, Lai J, Cai X, Zhang L, Wang S, Chen M, Cao W, Zhu Q, Gou H. Contributed data or analysis tools: Liu T, Li Q, Lin Z, Liu C, Pu W, Zeng S, Lai J, Cai X, Zhang L, Wang S, Chen M, Cao W, Zhu Q, Gou H. Performed the analysis: Liu T, Li Q, Lin Z, Zeng S, Zhu Q, Gou H. Wrote the paper: Liu T, Li Q, Lin Z, Zhu Q, Gou H. AcknowledgmentsThis work was supported by the 1-3-5 Project of Discipline Excellence Development of West China Hospital (ZYJC21042), Sichuan University for Qing Zhu.
Fig. 1.Patient enrollment and disposition. AEs, adverse events; GAC, nab-paclitaxel plus gemcitabine and cisplatin; OS, overall survival; PFS, progression-free survival. 
Fig. 2.Efficacy data from whole populations. (A) Progression-free survival of all treated patients. (B) Overall survival of all treated patients. (C) Best response of tumor lesions. (C) Best response of tumor lesions. (D) Change of tumor diameters. (E) Treatment features of whole populations. CI, confidence interval; mOS, median overall survival; mPFS, median progression-free survival. 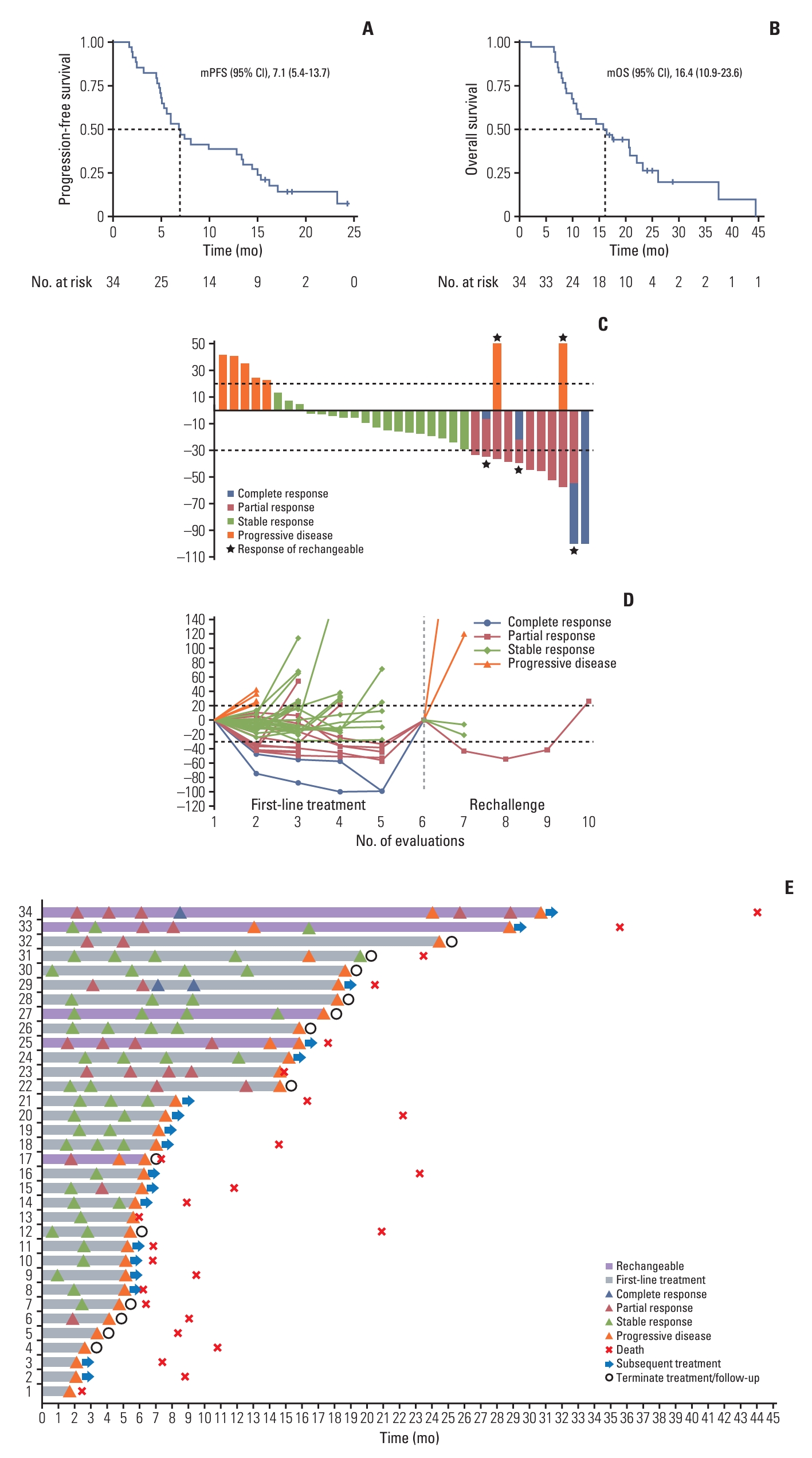
Fig. 3.Kaplan-Meier curves based on tumor markers. (A) Carbohydrate antigen (CA) 19-9 and carcinoembryonic antigen (CEA) baseline and post-treatment levels and their post-treatment changes in relation to progression-free survival. (B) Baseline and post-treatment levels of CA19-9 and CEA and their post-treatment changes in relation to overall survival. 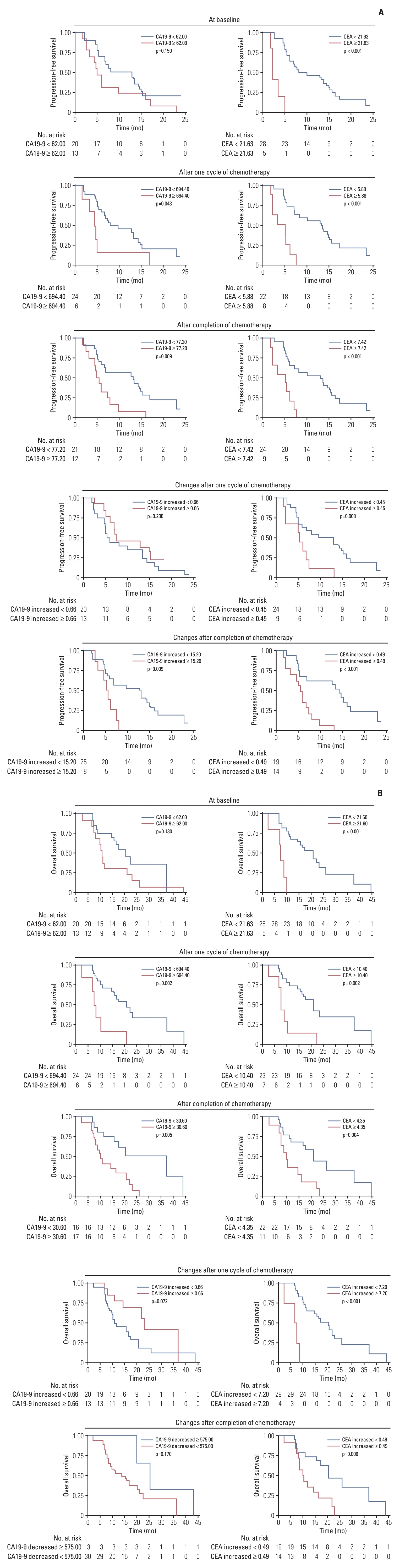
Fig. 4.Multiplex immunofluorescence analysis of tumor microenvironment (TME) before treatment. (A) Representative fluorescence images of a response patient illustrating the higher number of CD4+, CD8+ T-cell infiltrate before treatment. (B) Representative fluorescence images of a non-response patient illustrating the lower number of CD4+, CD8+ T-cell infiltrate and higher number of programmed death-1–positive (PD-1+), TIGIT+ T-cell infiltrate before treatment. (C-G) Comparison of different types of immune cells in the TME of response and non-response patients. *p < 0.05, **p < 0.01; ns, not significant. 
Table 1.Pretreatment patient characteristic
Table 2.Adverse events References2. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261–80.
3. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21:594–9.
4. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–89.
5. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81.
6. Komaya K, Ebata T, Shirai K, Ohira S, Morofuji N, Akutagawa A, et al. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br J Surg. 2017;104:426–33.
7. Komaya K, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, et al. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: analysis of a large cohort with a close postoperative follow-up approach. Surgery. 2018;163:732–8.
8. Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391–8.
9. Kim BJ, Hyung J, Yoo C, Kim KP, Park SJ, Lee SS, et al. Prognostic factors in patients with advanced biliary tract cancer treated with first-line gemcitabine plus cisplatin: retrospective analysis of 740 patients. Cancer Chemother Pharmacol. 2017;80:209–15.
10. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5:824–30.
11. Jung K, Park J, Jung JH, Lee JC, Kim J, Hwang JH. Real-world outcomes of gemcitabine, cisplatin, and nab-paclitaxel chemotherapy regimen for advanced biliary tract cancer: a propensity score-matched analysis. Gut Liver. 2022;16:798–805.
12. Ouyang G, Liu Q, Wu Y, Liu Z, Lu W, Li S, et al. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study 2017. Cancer. 2021;127:2238–50.
13. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
14. Sahai V, Catalano PJ, Zalupski MM, Lubner SJ, Menge MR, Nimeiri HS, et al. Nab-paclitaxel and gemcitabine as first-line treatment of advanced or metastatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4:1707–12.
15. You MS, Ryu JK, Choi YH, Choi JH, Huh G, Paik WH, et al. Therapeutic outcomes and prognostic factors in unresectable gallbladder cancer treated with gemcitabine plus cisplatin. BMC Cancer. 2019;19:10.
16. Lamarca A, Ross P, Wasan HS, Hubner RA, McNamara MG, Lopes A, et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst. 2020;112:200–10.
17. Kang J, Lee SH, Son JH, Lee JW, Choi YH, Choi JH, et al. Body mass index and weight change during initial period of chemotherapy affect survival outcome in advanced biliary tract cancer patients. PLoS One. 2018;13:e0195118
18. Bouillanne O, Dupont-Belmont C, Hay P, Hamon-Vilcot B, Cynober L, Aussel C. Fat mass protects hospitalized elderly persons against morbidity and mortality. Am J Clin Nutr. 2009;90:505–10.
19. Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014;99:999–1005.
20. Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861–70.
21. Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol. 2011;18:3579–85.
22. Peixoto RD, Renouf D, Lim H. A population based analysis of prognostic factors in advanced biliary tract cancer. J Gastrointest Oncol. 2014;5:428–32.
23. Wang YF, Feng FL, Zhao XH, Ye ZX, Zeng HP, Li Z, et al. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol. 2014;20:4085–92.
24. Park HS, Park JS, Chun YJ, Roh YH, Moon J, Chon HJ, et al. Prognostic factors and scoring model for survival in metastatic biliary tract cancer. Cancer Res Treat. 2017;49:1127–39.
|
|