AbstractPurposeAlthough obesity is associated with numerous diseases, the risks of disease may depend on metabolically healthy status. Nevertheless, it is unclear to whether metabolically healthy status affects risk of gastrointestinal (GI) cancer in general Chinese population.
Materials and MethodsA total of 114,995 participants who met the criteria were included from the Kailuan Study. The study participants were divided into four groups according to body mass index (BMI)/waist circumference (WC) and metabolic status. Incident of GI cancer (esophageal cancer, gastric cancer, liver cancer, biliary cancer, pancreatic cancer, and colorectal cancer) during 2006-2020 were confirmed by review of medical records. The Cox proportional hazard regression models were used to assess the association metabolically healthy status with the risk of GI cancer by calculating the hazard ratios (HR) and 95% confidence interval (CI).
ResultsDuring a mean 13.76 years of follow-up, we documented 2,311 GI cancers. Multivariate Cox regression analysis showed that compared with the metabolically healthy normal-weight group, metabolically healthy obese (MHO) participants demonstrated an increased risk of developing GI cancer (HR, 1.54; 95% CI, 1.11 to 2.13) by BMI categories. However, such associations were not found for WC category. These associations were moderated by age, sex, and anatomical site of the tumor. Individuals with metabolic unhealthy normal-weight or metabolic unhealthy obesity phenotype also have an increased risk of GI cancer.
ConclusionMHO phenotype was associated with increased risk of GI cancer. Moreover, individuals who complicated by metabolic unhealthy status have an increased risk of developing GI cancer. Hence, clinicians should consider the risk of incident GI cancer in people with abnormal metabolically healthy status and counsel them about metabolic fitness and weight control.
IntroductionWith the effects of changing lifestyles and an aging population, gastrointestinal (GI) cancer has been the leading cause of death and major public health problems in China as the morbidity and mortality rate increases [1]. According to the International Agency for Research on Cancer (IARC) in 2020, China has the largest number of incident cancer cases and deaths for liver cancer, esophageal cancer, and gastric cancer, comprising 1.21 million (two-thirds of the world’s total) newly diagnosed cases. Colorectal cancer in China has also rapidly risen, accounting for more than 40% of the world’s incidence in 2020 [2,3]. Obesity and smoking have been identified as well-established risk factors for cancer and cancer-related mortality [4]. It has been estimated that population attributable risk of GI cancer increased by 13.08% with high body mass index (BMI) in China in 2031 [5]. The situation of GI cancer prevention and control in China is not optimistic.
Obesity, which is often accompanied by several metabolic disorders, may mediate the harmful effect on related GI cancer through several metabolic pathways [6]. However, several studies have recently shown that not all obesity individuals were accompanied by obesity-related metabolic abnormalities, a different phenotype termed as metabolically healthy obese (MHO) [7]. This phenotype was once thought to a benign condition and characterized by the absence of cardiometabolic abnormalities, including insulin resistance, dyslipidemia, and hypertension despite excessive body fat accumulation. Previous cohort studies have shown that MHO phenotype is associated with a higher risk of cardiovascular disease (CVD) or cancer compared with individuals with metabolically healthy normal-weight (MHNW), although inconsistent results have also been reported [8,9]. Due to the lack of uniform criteria for defining MHO, the extent to which metabolically healthy but obese people are at a lower risk for cancer or have a lower risk for mortality, compared with the rest of obese people, is currently under debate.
BMI, waist circumference (WC), and waist-hip ratio (WHR) were used to define general and abdominal obesity in clinical practice, respectively. Previous studies found that distinct body shape phenotypes were differentially associated with the risk of overall cancer [10]. Evidence from large, prospective studies regarding MHO and risk of GI cancer is lacking, especially in the context of high incidence in China. In this study, we used data of the Kailuan Study, an ongoing prospective cohort, investigated associations of metabolically healthy obesity with GI cancer risk. We hypothesized that these MHO phenotype might have different risk by using different criterions for obesity. Furthermore, we examined whether the association differed by sex and age.
Materials and Methods1. Study participantsThe data were obtained from a health examination of employees of the Kailuan Company in the city of Tangshan, Hebei Province, north of China. Tangshan is situated about 90 miles southeast of Beijing and represents the overall Chinese population from a socio-economic perspective. Over the past few decades, Kailuan Group has developed a comprehensive company managing coal production, machine manufacture, transportation, chemical production, education and health care, etc. The Kailuan Study is an ongoing prospective community-based cohort study conducted in Tangshan, China. All participants in the Kailuan Study are employees and retirees of the Kailuan Group. Details of the study design and procedure have been described elsewhere [11]. At baseline, 125,246 participants were recruited, underwent clinical and laboratory examinations, and completed a questionnaire interview (June 2006 to December 2009) at 11 hospitals affiliated with the Kailuan Group. Subsequent examinations involving anthropometric, laboratory examinations, and self-reported questionnaires (including educational level, smoking, drinking, and so on) occurred approximately biennially. Participants were excluded if they had prevalent cancer (n=439), or missing data on BMI or WC at baseline (n=8,388), or missing data on fasting blood glucose (FBG), systolic blood pressure (SBP), diastolic blood pressure, triglyceride (TG), and high density lipoprotein-cholesterol (HDL-C) at baseline (n=1,425). Ultimately, a total of 114,995 participants were enrolled in the present syudy (Fig. 1).
2. Data collection and definitionsInformation on demographic and clinical characteristics (age, sex, lifestyle, and family history of cancer, etc.) were collected using a self-reported questionnaire, as detailed elsewhere [12]. Education level was classified as primary school or below, middle school, and high school or above. Smoking and drinking status were classified as current or not. Dietary salt intake was classified as low (< 6 g/day), intermediate (6-12 g/day), and high (> 12 g/day). Active physical exercise was defined as “> 4 times per week and 20 minutes at time.”
Elbow venous blood samples of 5 mL were collected into an anticoagulant tube containing EDTA between 7:00-9:00 am after overnight fasting for at least 8 hours, and the serum was collected after centrifugation at 3,000 ×g for 10 minutes. The supernatant was measured within 4 hours. All biochemical measurement including TG, HDL-C, low-density lipoprotein cholesterol, high-sensitive C-reactive protein (Hs-CRP), FBG, etc. was measured on the Hitachi 747 autoanalyzer (Hitachi, Tokyo, Japan).
3. Definition of metabolic health statusBody weight, height, and WC were reported by trained nurses according to the standard methods. BMI was calculated as weight divided by square of height (kg/m2). The obesity was defined according to categories of BMI (kg/m2) categorized using Chinese standards: normal weight (BMI < 28 kg/m2), and obese (BMI ≥ 28 kg/m2) [13]. For WC criteria, central obesity was defined as WC ≥ 90 cm for males and WC ≥ 85 cm for females [14].
Metabolic status was defined with reference to the JACC Health Promotion Series [15]. Metabolic unhealth status was diagnosed by the presence of any one of four components: (1) serum TG ≥ 150 mg/dL or drug treatment for elevated TG; (2) serum HDL-C < 50 mg/dL in women or < 40 mg/dL in men or drug treatment; (3) systolic blood pressure (BP) ≥ 130 mmHg or diastolic BP ≥ 85 mmHg or drug treatment for elevated BP; and (4) FBG ≥ 100 mg/dL or drug treatment for elevated FBG. Participants who meet 0 of the four components criteria were considered metabolic health status.
Using the above criteria for obesity and metabolic status, participants were categorized into four phenotypes: (1) MHNW, (2) MHO, (3) metabolic unhealthy normal-weight (MUNW), (4) metabolic unhealthy obesity (MUO). For WC criteria, all participants were also classified into four obesity phenotypes.
4. Assessment of GI cancerFollow-up ended at the first record of GI cancer event, all-cause death or at the end of follow-up on 31 December 2021, whichever came first. The types of GI cancer included esophageal cancer, gastric cancer, colorectal cancer, biliary tract cancer, liver cancer, and pancreatic cancer. We used International Classification of Diseases, 10th revision codes to identify GI cancer cases (C15 for esophageal cancer, C16 for gastric cancer, C18-C21 for colorectal cancer, C22 for liver cancer, C23-C24 for biliary tract cancer, and C25 for pancreatic cancer). In brief, participants with cancer were tracked through biennial health examinations and annual searches of the Tangshan medical insurance system and the Kailuan social security system. Moreover, the outcome information was further confirmed by a medical record review performed by clinical experts. Information on pathological diagnosis, imaging diagnosis (including ultrasonography, computerized tomography, and magnetic resonance imaging), and blood biochemical testing were collected to assess incident cancer.
5. Statistical analysisContinuous variables were compared using analysis of variance or the Kruskal-Wallis test according to distribution, and categorical variables were compared with the chi-square test.
Cumulative incidence rates were estimated by Kaplan-Meier survival analyses. Cox proportional hazard models were used with follow-up period as the time scale to estimate the hazard ratios for incident GI cancer and cancer type by metabolic health status, and were adjusted for baseline confounders, including age, sex, educational level, drinking (current or not), smoking (current or not), dietary salt intake (low, intermediate, and high), physical exercise (active or inactive), alanine aminotransferase, Hs-CRP and family history of cancer. Certain cancers are sex-specific and age-related. We performed interaction analyses to assess the interaction between metabolic health status and sex and age (≤ 60 years or > 60 years) on the risks of GI cancer. And the interaction effect was estimated by the Wald test.
To examine the robustness of our results, we performed several sensitivity analyses. First, we excluded events occurring in the first 1 year of follow-up to minimize potential reverse causation. Second, to weaken the influence of a treatment bias, we excluded participants with cardiovascular diseases at baseline and repeated analysis. Third, we also excluded participants received treatment with lipid lowering medication, hypoglycemic drug, or antihypertensive medication at baseline. Finally, we also assessed central obesity defined by WHR and waist-to-height ratio (WHtR). And sensitivity analysis was performed on the basis of the new definition of obesity used in the different criteria and metabolic status.
Missing covariates were imputed by multiple imputation using the fully conditional specification method SAS MI procedure. The results were consistent from analyses that excluded participants with missing covariates. The proportional hazard assumption was examined by Schoenfeld residuals. All analyses were done with SAS ver. 9.4 (SAS Institute Inc., Cary, NC), at a two-tailed alpha level of 0.05.
ResultsA total of 114,995 eligible participants were included in present analysis, their mean age was 51.08±12.63 years, and 80.34% were men. To baseline characteristics of the study participants according to metabolically healthy phenotypes based on BMI category are shown in Table 1. When compared to the MHNW group, participants in the MUNW and MUO groups were more likely to be older, men, a higher prevalence of current drinkers, had a higher BMI, WC, SBP, FBG, and TG level, and a lower HDL-C level. S1 Table shows the baseline characteristics when using WC for the classification of obesity.
After a mean follow-up of 13.76±2.74 years, incident GI cancer, esophageal cancer, gastric cancer, colorectal cancer, biliary tract cancer, liver cancer, and pancreatic cancer occurred in 2,311, 238, 492, 831, 75, 511, and 164, respectively. Table 2 shows the association between metabolically healthy phenotypes and incident GI cancer based on BMI category. In the multiple-adjusted regression analysis, MHO was positively associated with risk for GI cancer. However, such associations were not found for WC category. In addition, compared with the MHNW group, subjects from the MUNW and MUO groups had a higher risk of incident GI cancer among both BMI and WC category. The consistent results stratified by age and sex are displayed in Table 3. The detailed results of sensitivity analysis are reported in S2 and S3 Tables. In sensitivity analysis, we excluded participants with less than 1-year follow-up, with cardiovascular diseases at baseline, or received treatment with lipid lowering medication at baseline, the results were materially unchanged.
Fig. 2 shows the results of restricted cubic spline analysis. The dose-response relationship between baseline BMI and incident GI cancer was non-linear (p for non-linearity < 0.05) throughout the range of their levels in metabolically healthy participants. However, there was no dose-response relationship between BMI and GI cancer in metabolically unhealthy participants (p > 0.05). And no dose-response relationship was observed between WC and GI cancer regardless of metabolically healthy phenotypes (all p > 0.05).
Table 4 shows that the association between metabolically healthy phenotypes and GI cancer types. Significant differences were also evident for some types. MHO individuals were significantly associated with increased risk of gastric cancer using BMI category, while using WC criteria, individuals have increased risks for colorectal cancer and liver cancer. S3 Table shows that the association between metabolically healthy phenotypes (defined by WHR and WHtR categories) and GI cancer. Subgroup analyses found consistent results (S4-S7 Tables).
DiscussionIn this large population-based prospective cohort study and had a long follow-up period of up to 15years, we found an association between metabolically healthy status and risk of GI cancer from Kailuan Study. In our research, we first discovered that differences in the risk of developing GI cancer among different obesity phenotypes using different obese indicators for defining metabolically healthy status in Chinese, and the risk is primarily driven by gastric cancer, liver cancer, and colorectal cancer. We also found that, regardless of body fatness, metabolic disorders associated with increased GI cancer risk.
Scholars believe that MHO is a benign condition in studies in the early stages, who obese do not display the typical adverse metabolic effects of obesity [16,17]. However, current available evidence suggests MHO individuals are at increased risk for developing GI cancer despite normal metabolic traits including the parameters related to glucose and lipid metabolism, and BP. We provide robust evidence of a significant association of MHO with increased risk of GI cancer. In this study, we found that the MHO phenotype increased GI cancer risk during 15-year period compared with the MHNW participants, and the risk is primarily driven by gastric cancer, liver cancer and colorectal cancer. It is important to note that differences in the risk of developing GI cancer among different obesity phenotypes using different obese indicators for defining metabolically healthy status in Chinese. When grouped based on BMI, MHO had increased the risk of gastric cancer. In contrast, MHO individuals are at increased risk for developing liver cancer and colorectal cancer as assessed by using WC. Although BMI is commonly used as a standard measurement of overall adiposity in adults, the skeleton of Chinese population is relatively small, but the abdominal fat is relatively thick, which is one of the reasons that we considered multiple measures of adiposity [18]. When we analyzed the association between MHO and the risk of GI cancer, future studies are required to specifically define these criteria. According to age and sex, the metabolic status is quite different, and the common site-specific cancers are also quite different. In stratified analyses, the younger MHO participants had a higher risk of developing GI cancer compared with the older counterpart. From a life course perspective, early life BMI increase was likely a reflection of the life-long exposure to adiposity and adiposity-induced biologic alterations, which may result in greater impact cumulatively over decades of cancer development [19]. The findings we report in this large-scale study sometimes did not concur with prior, smaller studies [20,21]. This might be due to the following two reasons. On the one hand, there is no gold standard to define metabolically healthy status, inconsistent results have been obtained from different criteria. On the other hand, it takes years to decades for GI cancer development following MHO exposure, the follow-up time relatively short and the results may not necessarily represent long-term outcomes. This in part might also be related to ethnic disparities with the prevalence of GI cancer.
Another critical finding of this study is, regardless of body fatness, metabolic disorders associated with increased GI cancer risk. As shown in Fig. 2, individuals with metabolic abnormalities were at higher risks of GI cancer irrespective of degree of obesity. Although elevated BMI or WC are major risk factors for the development of metabolic disorders such as hypertension, diabetes, and dyslipidemia these metabolic abnormalities could also affect the risk of developing cancer independently, even in normal-weight individuals [22]. We also found that metabolic derangements that place an individual at increased risk of developing GI cancer are found in a proportion of normal-weight individuals (i.e., normal or overweight states), who have one or multiple metabolic abnormalities. Metabolic abnormality and the degree of obesity were more prominent in MUO individuals. As expected, the risk to developing GI cancer is higher in people with MUO compared to MUNW indicating that a potential interaction between obesity and metabolic abnormalities. This conclusion is supported by convergent evidence. In the EPIC prospective study, by using a case control study approach, Murphy et al found that individuals with the metabolically unhealthy/overweight phenotype (with hyperinsulinemia) are at higher colorectal cancer risk than those with normal insulin levels [23]. Concerning the concordance between MUO and the risk of GI cancer, this was also mentioned above [21,24].
As seen above, there are many inconsistencies on metabolically healthy status and risk of GI cancer, it makes it difficult to determine its exact relationship. The reason may be attributed rather to the lack of well-defined criteria to unambiguously define metabolically healthy status. Hence, as past literatures have reported, the impact of different diagnostic criteria on GI cancer suggests that patients with different diagnostic criteria might have different prevalence of disease [9]. That is why there are many non-congruent facts about metabolic healthy status and GI cancer in the literature. In this study, we defined metabolic healthy status according to Lavie criteria, which have been used to classify the risk of GI cancer in BMI categories [15]. And based on existing criteria, we complement many results that defined central obesity based on WC or WHtR. The same is true for the metabolic healthy status. Individuals were also diagnosed with metabolic healthy status if they met any of four diagnostic criteria for metabolic syndrome. Metabolic healthy status was not uniformly defined, which might result in obscure findings. Thus, we might have to be more careful in the selection of criteria. Several potential mechanisms have been identified in relation to the correlation between metabolically healthy phenotypes and the incidence and prognosis of GI cancer. One such mechanism involves chronic inflammation, characterized by alterations in concentrations of inflammatory cytokines and infiltration of immunosuppressive cells [25,26]. Furthermore, insulin resistance associated with metabolic unhealthiness, independent of general obesity, may elevate levels of insulin and insulin-like growth factor I, which are known to impede apoptosis and stimulate the proliferation of cancer cells [27,28]. Future studies are needed to clarify the underlying pathophysiological mechanisms of this association.
The strengths of this study include a cohort design, long follow-up, large sample size, and analysis using various criteria for obesity. However, this study also had several limitations. First, although we adjusted for important confounders in the multiple analysis, we cannot exclude the possibility of residual confounding factors due to unmeasured variables, such as dietary patterns, history of Helicobacter pylori infection or Clonorchis sinensis infection. Second, more evidence suggests that MHO is not a permanent state, but it may be a dynamic nature [29-32]. Approximately 30% to 50% of individuals originally identified as MHO was transitioned to a metabolic abnormality over time [33]. Regrettably, our analysis was based solely on baseline data. Future studies will require studies in focused the associations with the MHO phenotypic transitions and incident GI cancer. Last, our study population comprised participants with Kailuan Study, not covering completely Chinese.
In the present study, we observed that MHO phenotype was associated with increased risk of GI cancer, no matter general or abdominal obesity. Moreover, individuals who complicated by metabolic unhealthy status have an increased risk of developing GI cancer. Hence, clinicians should consider the risk of incident GI cancer in people with abnormal metabolically healthy status and counsel them about metabolic fitness and weight control.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The study was performed according to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Kailuan General Hospital (approval number: 2006-05). All participants were agreed to take part in the study and provided informed written consent. AcknowledgmentsThe authors thank all the members of the Kailuan Study Team for their contributions and the participants who contributed their data.
Fig. 1.Flowchart of the study population. BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL, high density lipoprotein; SBP, systolic blood pressure; TG, triglyceride; WC, waist circumference; WHR, waist-hip ratio; WHtR, waist-to-height ratio. 
Fig. 2.Dose-response relationship between body mass index/waist circumference level and gastrointestinal (GI) cancer. The association between body mass index and incident GI cancer among metabolically healthy (A) and unhealthy (B) participants, with reference BMI=28 kg/m2. The association between waist circumference level and incident GI cancer among metabolically healthy (C) and unhealthy (D) participants, with reference waist circumference=85 cm. The solid lines and shaded areas represent the hazard ratios and corresponding 95% confidence intervals. The models were adjusted for age, sex, educational level, drinking, smoking, physical exercise, family history of cancer, salt intake, high-sensitive C-reactive protein, and alanine transaminase. p-values for non-linearity were obtained using a chisquared test to compare nested models. 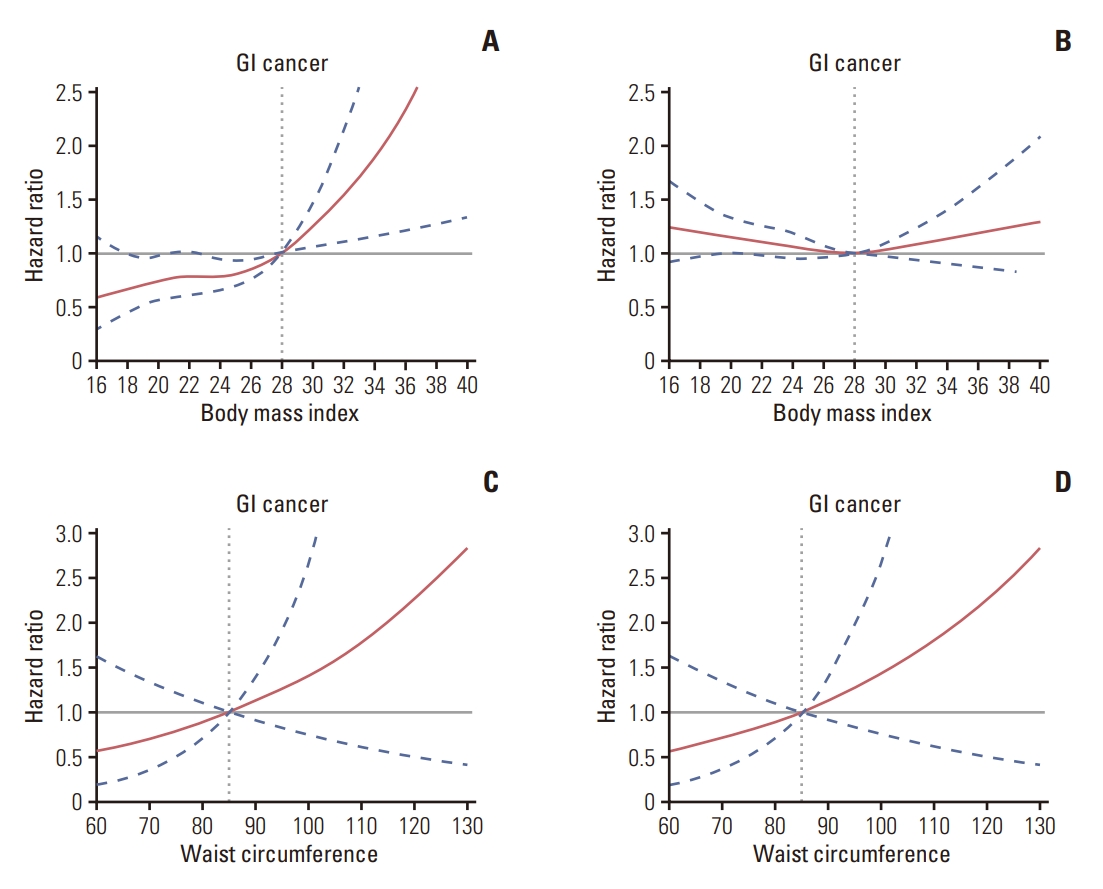
Table 1.Baseline characteristics of the study participants according to metabolically healthy phenotypes based on BMI category Values are presented as number (%), mean±SD, or median (P25-P75). ALT, alanine transaminase; BMI, body mass index; HDL-C, high density lipoprotein-cholesterol; Hs-CRP, high-sensitive C-reactive protein; MHNW, metabolic healthy normal-weight; MHO, metabolic healthy obesity; MUNW, metabolic unhealthy normal-weight; MUO, metabolic unhealthy obesity; SBP, systolic blood pressure; SD, standard deviation; TG, triglyceride; WC, waist circumference; WHR, waist-hip ratio; WHtR, waist-to-height ratio. Table 2.The association between metabolically healthy phenotypes (defined by BMI and WC categories) and GI cancer Multiple models adjusted for age, sex, educational level, drinking, smoking, physical exercise, family history of cancer, salt intake, highsensitive C-reactive protein, and alanine transaminase. BMI, body mass index; GI, gastrointestinal; MHNW, metabolic healthy normalweight; MHO, metabolic healthy obesity; MUNW, metabolic unhealthy normal-weight; MUO, metabolic unhealthy obesity; WC, waist circumference. Table 3.Subgroup analyses of the association between metabolically healthy phenotypes (defined by BMI and WC categories) and GI cancer Multiple models adjusted for age/sex, educational level, drinking, smoking, physical exercise, family history of cancer, salt intake, highsensitive C-reactive protein, and alanine transaminase. The interaction effect was estimated by the Wald test. Interaction between metabolically healthy phenotypes by BMI and age (p for interaction < 0.001) and sex (p for interaction < 0.001). Interaction between metabolically healthy phenotypes by WC and age (p for interaction < 0.001). And no interaction between metabolically healthy phenotypes by WC and sex (p for interaction > 0.05). BMI, body mass index; GI, gastrointestinal; MHNW, metabolic healthy normal-weight; MHO, metabolic healthy obesity; MUNW, metabolic unhealthy normal-weight; MUO, metabolic unhealthy obesity; WC, waist circumference. Table 4.The association between metabolically healthy phenotypes (defined by BMI and WC categories) and GI cancer type Multiple models adjusted for age, sex, educational level, drinking, smoking, physical exercise, family history of cancer, salt intake, high-sensitive C-reactive protein, and alanine transaminase. Model for colorectal cancer was additionally adjusted for high fat diet. Model for biliary tract cancer was additionally adjusted for gallstone. Model for liver cancer was additionally adjusted for hepatitis B virus surface antigen. Model for pancreatic cancer was additionally adjusted for history of chronic pancreatitis. BMI, body mass index; GI, gastrointestinal; MHNW, metabolic healthy normal-weight; MHO, metabolic healthy obesity; MUNW, metabolic unhealthy normal-weight; MUO, metabolic unhealthy obesity; WC, waist circumference. References1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584–90.
3. Wild CP, Weiderpass E, Stewart BW. World cancer report 2020: cancer research for cancer prevention [Internet. Lyon: International Agency for Research on Cancer; 2020. [cited 2021 Feb 22]. Available from: http://publications.iarc.fr/586
4. GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400:563–91.
5. Wu Y, Li Y, Giovannucci E. Potential impact of time trend of lifestyle risk factors on burden of major gastrointestinal cancers in China. Gastroenterology. 2021;161:1830–41.
6. Murphy N, Jenab M, Gunter MJ. Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol. 2018;15:659–70.
8. Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (NordTrondelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–8.
9. Lin CJ, Chang YC, Cheng TY, Lo K, Liu SJ, Yeh TL. The association between metabolically healthy obesity and risk of cancer: a systematic review and meta-analysis of prospective cohort studies. Obes Rev. 2020;21:e13049
10. Sedlmeier AM, Viallon V, Ferrari P, Peruchet-Noray L, Fontvieille E, Amadou A, et al. Body shape phenotypes of multiple anthropometric traits and cancer risk: a multi-national cohort study. Br J Cancer. 2023;128:594–605.
11. Cui H, Liu Q, Wu Y, Cao L. Cumulative triglyceride-glucose index is a risk for CVD: a prospective cohort study. Cardiovasc Diabetol. 2022;21:22.
12. Ma X, Cui H, Sun M, Liu Q, Liu X, Li G, et al. Fasting blood glucose, cholesterol, and risk of primary liver cancer: the Kailuan study. Cancer Res Treat. 2021;53:1113–22.
13. Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17 Suppl:1–36.
14. Zhou BF; Coorperative Meta-Analysis Group Of China Obesity Task Force. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96.
15. Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy weight and obesity prevention: JACC health promotion series. J Am Coll Cardiol. 2018;72:1506–31.
16. Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–16.
17. Stefan N, Schick F, Haring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017;26:292–300.
18. Pang Q, Zhang JY, Song SD, Qu K, Xu XS, Liu SS, et al. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J Gastroenterol. 2015;21:1650–62.
19. Huang T, Tworoger SS, Willett WC, Stampfer MJ, Rosner BA. Associations of early life and adulthood adiposity with risk of epithelial ovarian cancer. Ann Oncol. 2019;30:303–9.
20. Kabat GC, Kim MY, Stefanick M, Ho GYF, Lane DS, Odegaard AO, et al. Metabolic obesity phenotypes and risk of colorectal cancer in postmenopausal women. Int J Cancer. 2018;143:543–51.
21. Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Impact of metabolically healthy obesity on the risk of incident gastric cancer: a population-based cohort study. BMC Endocr Disord. 2020;20:11.
22. Park YM, White AJ, Nichols HB, O’Brien KM, Weinberg CR, Sandler DP. The association between metabolic health, obesity phenotype and the risk of breast cancer. Int J Cancer. 2017;140:2657–66.
23. Murphy N, Cross AJ, Abubakar M, Jenab M, Aleksandrova K, Boutron-Ruault MC, et al. A nested case-control study of metabolically defined body size phenotypes and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS Med. 2016;13:e1001988
24. Cao Z, Zheng X, Yang H, Li S, Xu F, Yang X, et al. Association of obesity status and metabolic syndrome with site-specific cancers: a population-based cohort study. Br J Cancer. 2020;123:1336–44.
25. Braun S, Bitton-Worms K, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci. 2011;7:1003–15.
26. Aguilar-Salinas CA, Garcia EG, Robles L, Riano D, Ruiz-Gomez DG, Garcia-Ulloa AC, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93:4075–9.
27. Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–8.
28. Liu T, Zhang Q, Wang Y, Ma X, Zhang Q, Song M, et al. Association between the TyG index and TG/HDL-C ratio as insulin resistance markers and the risk of colorectal cancer. BMC Cancer. 2022;22:1007.
29. Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6:714–24.
30. Gao M, Lv J, Yu C, Guo Y, Bian Z, Yang R, et al. Metabolically healthy obesity, transition to unhealthy metabolic status, and vascular disease in Chinese adults: a cohort study. PLoS Med. 2020;17:e1003351
31. Nam KH, Yun HR, Joo YS, Kim J, Lee S, Lee C, et al. Changes in obese metabolic phenotypes over time and risk of incident chronic kidney disease. Diabetes Obes Metab. 2018;20:2778–91.
|
|
|||||||||||||||||||||||||||||||||||||||