AbstractPurposeThe authors conducted a multicenter study to evaluate the efficacy and safety of combination chemotherapy with Padexol® and cisplatin for treating patients with advanced non-small cell lung cancer (NSCLC).
Materials and MethodsFrom November 2003 to April 2005, 42 chemo-naive patients with advanced NSCLC were enrolled into this study from 4 hospitals. The treatment consisted of Padexol® 175 mg/m2 as a 3-hr infusion, and this was followed by cisplatin 75 mg/m2 administered as an intravenous infusion with standard premedication. The treatment was repeated every 3 weeks.
ResultsAmong the 42 patients (pts), 33 pts were evaluable for response. On the per protocol analysis, 1 patient (pt) (3.0%) achieved complete response (CR), 17 pts (51.5%) achieved partial response (PR), 6 pts (18.2%) achieved stable disease (SD), and 9 pts (27.3%) progressed; therefore, the overall response rate was 54.6% (95% CI: 37.6~71.5%). On the intention-to-treat analysis, 1 pt (2.4%) achieved CR, 18 pts (42.9%) achieved PR, 11 pts (26.2%) achieved SD, and 9 pts (21.4%) progressed; therefore, the overall response rate was 45.2% (95% CI: 30.2~60.3%). The response, as evaluated by the investigators, was independently reviewed by 2 external radiologists and it was as follows; 13 PR (43.3%), 14 SD (46.7%) and 3 progressive disease (10%). The median duration of response was 5.9 months. The median follow-up duration was 10.3 months (range: 1.3 to 22.1 months). The median time to progression was 5.8 months (95% CI: 4.7 to 7.4 months). The median survival time on the intention-to-treat analysis was 10.5 months (95% CI: 8.1 to 18.8 months). The most common grade 3 or 4 hematologic toxicities were neutropenia (26/180 cycles, 14.4%), anemia (7/180 cycles, 3.9%) and febrile neutropenia (2/180 cycles, 1.1%). The most frequent grade 3 or 4 non-hematologic toxicities were nausea (14/42 patients, 14.3%), anorexia (3/42 patients, 7.1%) and myalgia (3/42 patients, 7.1%).
INTRODUCTIONNon-small cell lung cancer (NSCLC) is the most common cause of cancer death and the second common cancer following gastric cancer in Korea. According to the lung cancer registry of St. Vincent's Hospital, NSCLC accounted for 82.3% of all lung cancer, but only 27.1% of NSCLC was operable (1).
Platinum-based combination chemotherapy with using paclitaxel, gemcitabine, docetaxel or carboplatin has shown high response rates and a survival benefit for patients with advanced NSCLC (2,3), and paclitaxel and cisplatin combination chemotherapy is commonly used in patients with NSCLC (4,5).
Paclitaxel (Taxol®) is a novel anticancer agent extracted from the bark of the Pacific yew tree (Taxus brevifolia); it is formulated in Cremophor EL, and it has a unique antitumor activity that involves the promotion and stabilization of microtubule assembly (6). Paclitaxel has a broad antitumor activity against NSCLC, breast cancer, ovary cancer, bladder cancer and other cancers.
Padexol® is formulated in Acephorol 330 as a solubilizer, which is similar to Cremophor EL (polyethoxylated castor oil) (7). The chemical structure and molecular weight of Padexol are the same as those of paclitaxel and the anticancer effects of Padexol® are similar to Taxol®, as was tested for both in vitro and in vivo. Padexol® has been approved by the Korean FDA for use in ovary, breast and gastric cancers and NSCLC.
We conducted a multicenter study to evaluate the efficacy and safety of combination chemotherapy of Padexol® and cisplatin for treating patients with advanced NSCLC.
MATERIALS AND METHODS1) Patient populationThe eligible patients were between 18 and 75 years of age, and they suffered with histologically confirmed NSCLC that was inoperable or recurrent after surgery/radiotherapy. Prior radiotherapy was permitted if it had been done at least 4 weeks before entry into the study. The patients should have a ECOG performance status of 0~2, at least 1 clinically or radiographically measurable lesion greater than 10 mm in diameter or a CT-scan/Sonographically measurable lesion greater than 20 mm in diameter according to the RECIST criteria, normal hematologic values (neutrophils ≥1.5×109/L and a platelet count ≥100×109/L), an adequate hepatic function (serum bilirubin ≤1.25×the upper normal limit (UNL); ALT and AST ≤3.0×UNL), an adequate renal function (serum creatinine ≤1×UNL) and a life expectancy of at least 3 months. The patients should not have a prior chemotherapy history, a history of other carcinomas, any prior unanticipated severe reaction to the polyethylated caster oil-containing agents, clinically significant cardiac disease, evidence of CNS metastases, symptomatic peripheral neuropathy ≥grade 2, congestive heart failure, myocardial infarction within 6 months before enrollment, active infections or any other underlying medical condition that would interfere with their participation in the study. All the patients were required to give a written informed consent.
2) Treatment scheduleThe treatment consisted of Padexol® 175 mg/m2 as a 3-hr infusion, and this was followed by cisplatin 75 mg/m2 administered as an IV infusion with a standard premedication and hydration method on the first day of each 3-week treatment cycle. Patients with clearly documented progressive disease were discontinued from further study treatment. The patients who responded to treatment or whose disease was stable were scheduled to receive at least 4 cycles and they were treated until they finished 6 cycles unless they experienced intolerable toxicity or the patients withdrew from the study.
3) Dose modificationsThe toxicity was evaluated before each treatment cycle according to the NCI CTC version 2.0. If the hematopoietic function had not recovered by the first day of the next cycle (neutrophil <1.5×109/L), then the administration of Padexol® and cisplatin was delayed for a maximum of 2 weeks. For the patients that experienced grade 4 neutropenia or thrombocytopenia, grade 3 mucositis or grade 2 peripheral neuropathy, the dosage of Padexol® and cisplatin was reduced by 20% for the next cycle. If the above toxicities occurred after dose reduction, the dose of Padexol® was reduced by an additional 20%. Administration of the drugs was discontinued if the patient showed major organ toxicity (cardiovascular, nephro- or hepato-toxicity) of grade 3 or other major toxicities (excluding nausea, alopecia and vomiting) of grade 3 that were not reversible within 2 weeks after dose reduction.
4) Study assessmentsPhysical examinations, complete blood counts and the relevant biochemical tests were performed for all the patients before each cycle of therapy. Imaging studies, including CT scan, were carried out at the baseline, after every 2 cycles of therapy and when there was any clinical suspicion of disease progression. The tumor responses were categorized as complete, partial, progressive or stable based on the standard RECIST criteria. An objective response required one confirmatory follow-up scan at least 4 weeks after the first documentation of the response. The patients who discontinued or completed the study were evaluated at least every 3 months. The patients were considered assessable for their treatment response if they had early disease progression or if they had received at least 4 cycles of treatment with at least 2 tumor assessments. The tumor measurement was evaluated by an investigator from each institute and the measurements were independently reviewed again by 2 external radiologists. The standard treatment efficacy end points in relation to survival, the objective response, time to progression (TTP) and the duration of response were determined. Duration of response was defined as the time from the first confirmed date of partial or complete response until the first confirmed date of disease progression for the patients with a partial or complete response. The TTP was measured from the start of the treatment until disease progression for all the subjects. Overall survival (OS) was measured from the start of the treatment until death. Toxicity was graded according to the NCI CTC.
5) Statistical analysisThe trial was conducted according to the group sequential design that was suggested by Chang et al. with the response rate as the primary end point (8). The number of patients required for the study was calculated based on a targeted response rate of 37% and a minimum response rate of 20%, with α error of 0.0441 and β error of 0.1728. Therefore, the required number of patients was 50. The interim analysis was carried out when the first 30 assessable patients had been recruited. The trial would be evaluated if 11 or more responses were observed with the conclusion being that the regimen was sufficiently active to be submitted for further study.
The analysis of the clinical efficacy was performed on the per protocol basis and according to an intent-to-treat analysis. Kaplan-Meier estimates were used in the analysis of all the time-to-event variables (duration of response, time to progression and the overall survival), and the 95% confidence interval (CI) for the median time to the event was computed. The SAS version 8.1 for Windows was used for the statistical computation.
RESULTSFrom November 2003 to April 2005, 42 patients were enrolled onto the study from 4 hospitals. All the patients received the test drug for more than 1 cycle. All the patients were assessable for safety and 33 patients were assessable for response. Fifteen patients discontinued the study before 4 cycles. One patient progressed after 1 cycle, 4 patients progressed after 2 cycles and 1 patient progressed after 3 cycles. Three patients withdrew their informed consent after 3 cycles. Three patients had their treatment discontinued due to serious adverse events. Two patients died after 2 or 3 cycles. One patient had treatment discontinued due to treatment delay.
1) Patient demographicsThe patient characteristics are listed in Table 1. Their median age was 63 years (range: 47~73 years), and there were 35 males and 7 females. Almost all the patients had a performance status of 0 to 1. 52% of the patients had non-squamous cell carcinoma, and 23 patients had stage IV disease.
2) Treatment administrationA total of 180 treatment cycles were administered, with a median of 4 cycles per patient (range: 1~6 cycles). Eighteen patients received 6 cycles and 27 patients received at least 4 cycles. The dose of Padexol® was reduced in 26 cycles and that of cisplatin was reduced in 33 cycles. Thus, the actual administered dose of Padexol® was 170.36 mg/m2/cycle and that of cisplatin was 72.55 mg/m2/cycle. Neutropenia, mucositis, nephrotoxicity and neuropathy were the common reasons for the dose reductions.
3) Efficacy and survivalAmong the 42 patients who received the study drugs, 33 patients were evaluable for response. On the per protocol analysis, 1 patient (3.0%) achieved a complete response (CR), 17 patients (51.5%) achieved a partial response (PR), 6 patients (18.2%) achieved stable disease (SD), and 9 patients (27.3%) progressed; therefore, the overall response rate was 54.6% (95% CI: 37.6~71.5%). On the intention-to-treat analysis, 1 patient (2.4%) achieved CR, 18 patients (42.9%) achieved PR, 11 patients (26.2%) achieved SD, and 9 patients (21.4%) progressed; therefore, the overall response rate was 45.2% (95% CI: 30.2-60.3%)(Table 2).
The response that was evaluated by the investigators was then reviewed by 2 external radiologists who were working independently. Three patients among the 9 patients with progressive disease (PD), as evaluated by the investigators, were categorized as non-evaluable. Thus 30 patients were reviewed for their responses. There were 13 PRs (43.3%), 14 SDs (46.7%) and 3 PDs (10%).
For the 18 responders, the median duration of response was 5.9 months. The median follow-up duration was 10.3 months (range: 1.3~22.1 months). The median survival time on the intention-to-treat analysis was 10.5 months (95% CI: 8.1~18.8 months). The Kaplan-Meier estimate of survival is shown in Fig. 1. The median TTP on the intention-to-treat analysis was 5.8 months (95% CI: 4.7~7.4 months) (Fig. 2).
4) SafetyAll the registered patients were assessable for the treatment-related adverse events and toxicities that were observed during the study, which are listed in Table 3 and 4. The most common grade 3 or 4 hematologic toxicities were neutropenia (26/180 cycles, 14.4%), anemia (7/180 cycles, 3.9%) and febrile neutropenia (2/180 cycles, 1.1%). The most frequent grade 3 or 4 non-hematologic toxicities were nausea (14/42, 14.3%), anorexia (3/42, 7.1%) and myalgia (3/42, 7.1%).
DISCUSSIONCisplatin-based combination chemotherapy has shown high response rate and survival benefit for the patients with stage III and IV NSCLC, but none of combination chemotherapy regimens (paclitaxel+cisplatin vs gemcitabine+cisplatin vs docetaxel+cisplatin vs paclitaxel+carboplatin) have shown a significant advantage over the others for the treatment of advanced NSCLC (9).
Paclitaxel and cisplatin combination chemotherapy is one of the most commonly used regimens for patients with NSCLC. The major toxicities of paclitaxel are hypersensitivity, myalgia and peripheral sensory neuropathy.
The efficacy and safety of a combination of Padexol® and cisplatin for the treatment of advanced gastric cancer have been studied in Korea (10). The overall response rate was 33% with a median overall survival time of 6.7 months, which were very similar to the results of other studies. The grade 3/4 toxicities were neutropenia (33% of patients), anemia (17.9% of patients), and anorexia (10.2% of patients). The incidence of neurotoxicity was only 2.5% (all grade 1/2 only). Kim et al. have concluded the combination of Padexol® and cisplatin was found to be active and it seems to be a relatively well-tolerated regimen for the treatment of advanced gastric cancer (10).
We conducted a multicenter study to evaluate the efficacy and safety of combination chemotherapy of Padexol® (175 mg/m2 as a 3-hr infusion) and cisplatin (75 mg/m2) for patients suffering with advanced NSCLC, and we compared it with the other previous results (7,8,10) of paclitaxel and cisplatin combination chemotherapy. von Pawel, et al (4) treated 75 patients with Taxol® (paclitaxel) and cisplatin with the same dose and schedule as our study. Lee, et al (5) treated 74 Korean patients with Taxol® (paclitaxel) and cisplatin at the same dose but with a different schedule, i.e., cisplatin was given on day 2. Lee, et al (11) treated 25 Korean patients with Genexol® (paclitaxel) and cisplatin at the same dose and schedule as our study (Table 5).
In this study, the response was reviewed by an independent external reviewer to ensure the validity of the data. We observed an overall response rate of 54.5% (1 CR and 17 PR in 33 patients) on the per protocol analysis and an overall response rate of 46.32% (1 CR and 18 PR in 42 patients) on the intention-to-treat analysis with a median duration of response of 5.9 months. The overall response rate, as evaluated by the external reviewer, was 43.3% in 30 patients. The response rate we observed in this study (54.5%) was quite similar to the response rate of other previous studies, i.e. 42% by von Pawel, et al. (4); 51% by Lee, et al (5) or higher (Table 5). The response rate, as evaluated by the independent reviewers, was not different from those evaluated by the investigators.
We observed the median time to progression and the median survival time were 5.9 months and 10.5 months, respectively, on the intention-to-treat analysis. The survival time we observed in this study was a little shorter than those observed in other studies, i.e. 13.7 months by Lee, et al (5) and 13.3 months by Lee, et al (11).
The most common grade 3 or 4 hematologic toxicities were neutropenia (14.4% of 180 cycles, 23.5% of patients) and anemia (3.9% of 180 cycles, 9.6% of patients), but the incidence of febrile neutropenia was only 1.1% of 180 cycles (4.8% of patients) in this study. von Pawel et al reported that the incidence of grade 3 and 4 neutropenia was 37% of 328 cycles (4). Lee, et al (5) reported a lower incidence of grade 3 or 4 neutropenia (0% of 327 cycles), anemia (2.4%), and febrile neutropenia (0.9%), while Lee, et al (11) reported a similar incidence of grade 3 or 4 hematologic toxicity in their study, i.e. grade 3 or 4 neutropenia in 10% of 106 cycles, anemia in 4% and febrile neutropenia in 1% of the cycles.
The most common non-hematologic toxicities in this study were nausea, neuropathy, maylagia, anorexia and arthralgia. The incidence of grade 3 myalgia was rare (1.7% of 180 cycles), and there was no grade 4 myalgia. Lee, et al reported 14 patients (18.9%) with grade 1 myalgia, 17 patients (23.0%) with grade 2 myalgia and 3 patients with grade 3 myalgia (4.1%) in their study (5). Genexol® and cisplatin combination chemotherapy induced grade 1 and 2 myalgia in 40% and 24%, respectively (11).
The incidences of grade 1 and 2 peripheral neuropathy were 30.0% and 7.8%, respectively, but grade 3 peripheral neuropathy was very rare (1.1%), and there was no patient with grade 4 neuropathy in this study. van Pawel et al reported 26% of grade 1 or 2 and 1% of grade 3 or 4 peripheral neuropathy (4). Lee at al (5) reported 33.8% with grade 1, 17.6% with grade 2 and 2.7% with grade 3 peripheral neuropathy. Genexol® and cisplatin combination chemotherapy induced grade 1 and 2 peripheral neuropathy in 48% and 20% of the patients, respectively (11). There was no patient with any hypersensitivity reaction.
The weak point of this study is that this study was not a randomized trial comparing Taxol® and cisplatin combination chemotherapy.
CONCLUSIONSIt is very difficult to directly compare our data with the other previously reported data of pacitaxel and cisplatin combination chemotherapy at the same dose, but the authors observed that Padexol® was as good as the other paclitaxel formulations (Taxol® or Genexol®) when combined with cisplatin for treating patients with NSCLC.
ACKNOWLEDGEMENTThis study was possible with help of the sub-investigators listed below. Hyun Sook Park, Study coordinator & Eun Young Kwon, Pharmacist of St. Vincent's Hospital; Kyung Wook Hur, Study coodinator & Yoon Jung Yeo, Pharmacist of Korean Univ. Medical Center; Myo Soon Kim, Study coodinator & Bo-Kyung Moon, Pharmacist & Mi-Joung Eun, Pharmacist of Daegu Catholic Univ. Medical Center; Jung-Eun Lee, Study coordinator & Myong Hee Ko, Pharmacist of Daegu Fatima Hospital.
References1. Jo HJ, Kim HK, Song SH, Kim CH, Cho KD, Cho DG, et al. Two Years' result of lung cancer registry in St. Vincent's Hospital. Proc Cancer Reser Treat. 2002;34(Suppl 1):143(#190)
2. Sandler AB, Ansari R, McClean J, Fisher W, Dorr A, Einhorn LH. A Hoosier Oncology Group phase II study of gemcitabine plus cisplatin in non-small cell lung cancer. Prog Proc Am Soc Clinc Oncol. 1995;14:357(abstract)
3. Langer CJ, Leighton JC, Comis RL, O'Dwyer PJ, McAleer CA, Bonjo CA, et al. Paclitaxel and carboplatin in combination in the treatment of advanced non-small-cell lung cancer: a phase II toxicity, response, and survival analysis. J Clin Oncol. 1995;13:1860–1870. PMID: 7543559
4. von Pawel J, Wagner H, Niederle N, Heider A, Koschel G, Hecker D, et al. Phase II study of paclitaxel and cisplatin in patients with non-small cell lung cancer. Semin Oncol. 1996;23(6):Suppl 1647–50. PMID: 9007121
5. Lee JA, Lee KS, Ahn JS, Byun JH, Song HH, Zang DY, et al. A phase II study of paclitaxel and cisplatin combination chemotherapy in advanced non-small cell lung cancer. Cancer Res Treat. 2003;35:239–244.
6. Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82:1247–1259. PMID: 1973737
7. Loos WJ, Szebeni J, ten Tije AJ, Verweij J, van Zomeren DM, Chung KN, et al. Preclinical evaluation of alternative pharmaceutical delivery vehicles for paclitaxel. Anticancer Drugs. 2002;13:767–775. PMID: 12187334
8. Chang MN, Therneau TM, Wieand HS, Cha SS. Designs for group sequential phase II clinical trilas. Biometrics. 1987;43:865–874. PMID: 3427171
9. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. PMID: 11784875
Fig. 1Overall survival. The Kaplan-Meier estimate of median overall survival was 10.5 months (95% CI, 8.1 to 18.8 months). 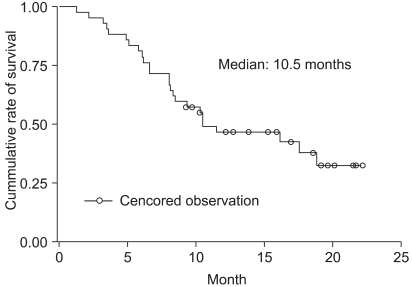
Fig. 2Time to disease progression. The Kaplan-Meier estimate of median time to disease progression was 5.8 months (95% CI, 4.7 to 7.4 months). 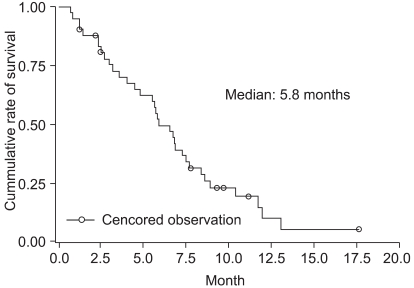
Table 2Clinical response 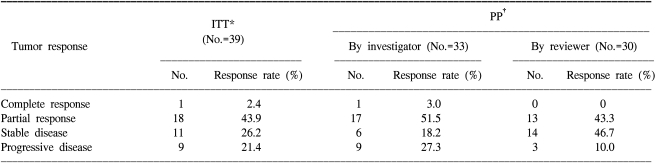 *Intention-to-treat analysis, †Per protocol analysis. Of the 42 eligible patients, 3 patients were not evaluable for ITT analysis. One developed G3 dyspnea after the 1st cycle, 1 died suddenly after full recovery from the 2nd cycle, and 1 was late for the 2nd cycle. There were patients who were not evaluable for PP analysis: 3 withdrew their consent after the 3rd cycle, 1 died of massive hemoptysis after the 3rd cycle, 1 stopped after the 3rd cycle due to G3 neuropathy and 1 developed pneumonia after the 2nd cycle. |
|
||||||||||||||||||||||||||||||||||||