AbstractPurposeA causal relationship between diabetes mellitus (DM) and pancreatic cancer is well established. However, in patients with advanced pancreatic cancer (APC) who receive palliative chemotherapy, the impact of DM on the prognosis of APC is unclear.
Materials and MethodsWe retrospectively enrolled APC patients who received palliative chemotherapy between 2003 and 2010. The patients were stratified according to the status of DM, in accordance with 2010 DM criteria (American Heart Association/American Diabetes Association). DM at least 2 years’ duration prior to diagnosis of APC was defined as remote-onset DM (vs. recent-onset).
ResultsOf the 349 APC patients, 183 (52.4%) had DM. Among the patients with DM, 160 patients had DM at the time of diagnosis of APC (remote-onset, 87; recent-onset, 73) and the remaining 23 patients developed DM during treatment of APC. Ultimately, 73.2% of patients (134/183) with DM received antidiabetic medication, including metformin (56 patients, 41.8%), sulfonylurea (62, 45.5%), and insulin (43, 32.1%). In multivariate analysis, cancer extent (hazard ratio [HR], 1.792; 95% confidence interval [CI], 1.313 to 2.445; p < 0.001) showed association with decreased overall survival (OS), whereas a diagnosis of DM (HR, 0.788; 95% CI, 0.615 to 1.009; p=0.059) conferred positive tendency on the OS. Metformin treatment itself conferred better OS in comparison within DM patients (HR 0.693; 95% CI, 0.492 to 0.977; p=0.036) and even in all APC patients (adjusted HR, 0.697; 95% CI, 0.491 to 1.990; p=0.044).
IntroductionPancreatic cancer (PC) is the fourth common cause of cancer death in both Asian and western countries [1]. At the time of diagnosis, only 10%-20% of patients are eligible for curative surgery. PC is an aggressive malignancy with a 5-year survival rate < 5% [2].
Diabetes mellitus (DM) is linked as a risk factor in a number of cancers [3]. Although the mechanisms of increased incidence of cancer with DM are not fully understood, insulin resistance, leading to hyperinsulinemia, causes up-regulation of insulin-like growth factor-I (IGF-I) signaling pathway to increase proliferation and invasion of cancer cells and decrease apoptosis [4,5].
Previous studies have shown that recent-onset DM has increased the risk of PC [6,7]. Some studies have even suggested that recent-onset DM could be used as a biomarker of asymptomatic early PC [8]. Although there is some controversy regarding whether DM predisposes to PC or is simply a consequence thereof, there is increasing evidence for recent-onset DM as an early manifestation of PC and for the long standing DM as a risk factor of PC with twice the incidence [9].
The utility of DM as a prognostic index in PC is a matter of debate. In two previous studies where patients with resectable and unresectable PC were grouped together, the survival was shortened for those with DM [10,11]. A retrospective review, limited to resectable PC, also showed that survival in patients with DM (especially recent-onset DM) was shortened [12]. Another retrospective study with 540 patients with any stage of PC reported that patients with DM alone without other constitutional symptoms such as pain, jaundice, and appetite loss had better survival than symptomatic patients [13]. However, for patients with unresectable PC receiving palliative chemotherapy, the prognostic value of DM has rarely been addressed despite the fact that this population constitutes the majority (approximately 80%) of patients with PC.
Recently, metformin, a widely used antidiabetic drug, has received attention for its anti-neoplastic role. Recent epidemiologic evidence is in favor of its protective role against PC [14] and of its beneficial roll in non-metastatic disease [15].
Therefore, we investigated whether DM and metformin have any association with survival of advanced PC (APC) patients undergoing palliative chemotherapy.
Materials and MethodsConsecutive patients with APC, confirmed by histology and all recipients of palliative chemotherapy from January 2003 to June 2010, were enrolled in this study.
1. Data collection and categorizationPrecisely defined DM disease status was a requirement, stipulated as follows: (1) self-reported diabetic history; (2) ongoing treatment with antidiabetic medication(s); or (3) qualification as DM during the follow-up period, based on 2010 American Heart Association/American Diabetes Association (AHA/ADA) joint criteria.
Each patient’s medical records were reviewed retrospectively. Date of diagnosis was the date that locally advanced PC (LAPC) or metastatic/recurrent PC (MPC) was confirmed. Patient age, performance status, DM status, antidiabetic treatments, body weight, body mass index (BMI), smoking history, and initial laboratory data were assessed.
For our purposes, we subdivided DM by onset and duration, designating diagnosis within 2 years prior to confirmed APC as “recent-onset DM,” and diagnosis > 2 years prior to confirmed APC as “remote-onset DM.” In addition, “preexisting DM” indicated diagnosis of DM before or at the time APC was confirmed (i.e., both “recent-onset” and “remote-onset” DM subsets) and “subsequent DM” corresponded with DM diagnosed after confirmation of APC. “Concurrent DM” incorporated both “preexisting” and “subsequent” subsets of DM.
Body weight and BMI at the time of initiation of palliative chemotherapy were cited as “initial weight” and “initial BMI.” “Initial weight loss” was the weight change experienced at the time of APC diagnosis, using a prior healthy state as reference. BMI was calculated individually as body weight divided by square of height (kg/m2). Patients were then stratified by BMI (< 22.5, 22.5-24.9, and ≥ 25.0), given the known association of BMI with mortality in Asians [16].
2. Statistical analysisThe study endpoint is overall survival (OS) defined as the period from diagnosis of APC to death from any cause. We calculated median OS using the Kaplan-Meier method. Between-group differences in demographic and clinical data were evaluated using Fisher exact test for categorical variables. For the effect of multiple factors on survival, the hazard ratio (HR) and its 95% confidence interval (CI) were evaluated using Cox proportional hazards model. The survival of the two groups was compared using the log-rank test. All tests were 2-sided, and p-values of ≤ 0.05 were considered statistically significant. We performed statistical analyses using SPSS ver. 19.0 (IBM Co., Armonk, NY).
This study was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-1102-023-350). All studies were conducted according to guidelines (Declaration of Helsinki) for biomedical research.
Results1. Patient characteristicsA total of 349 patients were enrolled. By definition, 183 patients (52.4%) had concurrent DM, whether preexisting (160 patients) or subsequent (23 patients). Of those with preexisting DM, 87 qualified as remote-onset and 73 as recent-onset. The majority of patients with concurrent DM (134/183, 73.2%) were taking antidiabetic medication, including metformin (56/134, 41.8%), sulfonylurea (62/134, 45.5%), and insulin (43/134, 32.1%) (Table 1). Median age of patients was 59.6 years and 64.2% were male. Sixty-six patients (18.9%) were diagnosed as LAPC and 283 patients (81.1%) as MPC. Four of every five subjects had good Eastern Cooperative Oncology Group performance status (0-1). Distribution of BMI was initially biased towards low (< 22.5 kg/m2, 62.0%; 22.5-24.9 kg/m2, 25.8%; ≥ 25.0 kg/m2, 12.2%) and one of every three patients (33.1%) suffered weight loss during the first-line chemotherapy. As a first-line chemotherapy, 324 patients (92.8%) received gemcitabine containing regimens and 53 patients (15.2%) were treated with gemcitabine or fluorouracil based concurrent chemoradiotherapy. Overall, 197 patients (56.4%) achieved favorable responses (complete response, partial response, and stable disease) to first-line palliative chemotherapy (Table 2).
Median duration of follow-up for all patients was 10.2 months (95% CI, 9.3 to 11.1 months), and median OS was 7.9 months (95% CI, 7.0 to 8.8 months). One-year and 2-year survival rates were 31% and 8%, respectively.
2. Determinants of prognosis for all patientsIn univariate analysis, Eastern Cooperative Oncology Group performance status (ECOG PS) (p=0.013), cancer extent (p < 0.001), concurrent DM (p=0.041), and weight loss during chemotherapy (p=0.026) significantly impacted OS (Table 3). In multivariate analysis, cancer extent/metastasis (HR, 1.792; 95% CI, 1.313 to 2.445; p < 0.001) conferred a higher risk of death, whereas concurrent DM (HR, 0.788; 95% CI, 0.615 to 1.009; p=0.059) showed the tendency of lowering risk.
3. Patient comparisons (DM vs. non-DM)The OS of the DM group was 8.4 months (95% CI, 6.8 to 10.0 months), compared with 7.5 months (95% CI, 6.3 to 8.7 months) for the non-DM group (p=0.041, log-rank test) (Fig. 1). The clinical characteristics of patients with APC were also compared in order to determine other factors causing the difference of OS between DM and non-DM subsets (Table 2). In the DM group, more patients were past the age of 60 years than in the non-DM group (59.6% vs. 38.0%, p < 0.001). ECOG PS, cancer extent, cancer antigen 19-9 level, and response to chemotherapy, etc. did not differ significantly between groups, although patients with DM showed a tendency for greater weight loss at diagnosis (change in BMI ≥ 1, p=0.067).
4. Analysis of OS in DM groupMore than half of our cohort had DM. When stratified as remote- or recent-onset DM, the clinical characteristics by subset were similar, except for more liberal use of antidiabetic medication in patients with remote-onset DM (p=0.002) (Supplementary Table 1). Metformin usage did not differ for remote-onset DM (32.2%) and recent-onset DM (34.2%), but insulin was more often used for remote-onset DM (34.5% vs. 17.8%, p=0.001).
Recent-onset DM did not have significantly prolonged OS, compared with remote-onset/subsequent DM (7.9 months vs. 9.8 months; HR, 0.789; 95% CI, 0.574 to 1.083; p=0.142) (Table 4, Fig. 2A). Neither antidiabetic medication in general nor sulfonylurea or insulin specifically affected OS in DM patients. However, recipients of metformin survived longer than non-recipients (HR, 0.693; 95% CI, 0.492 to 0.977; p=0.036) (Table 4, Fig. 2B). However, recipients and non-recipients of metformin had similar clinical characteristics (Supplementary Table 2).
5. Analysis of OS in all patients, relative to metformin useFocusing on metformin treatment, we reanalyzed prognostic indices of APC for the entire cohort, including non-DM patients (Table 5). The OS of metformin-treated patients was significantly longer than that of all other groups. Relative to the APC cohort overall, metformin treatment conferred better survival as well (11.0 months vs. 7.8 months; adjusted HR, 0.697; 95% CI, 0.491 to 1.990; p=0.044), given similar baseline clinical characteristics (Fig. 3A, Supplementary Table 3). OS of metformin-treated DM patients was also significantly longer than that of all other groups, respectively (non-DM or DM without metformin, p=0.029) (Fig. 3B).
DiscussionIn this study, we confirmed the possibility of metformin as an independent prognostic factor for APC patients undergoing palliative chemotherapy. Although we could not prove the association of DM with survival, we were able to show that APC patients with DM tend to survive longer than those without DM.
Metformin is an oral biguanide widely used as a single and combination therapy for DM. Many clinical data have proven the biological mechanisms of metformin as a glucose-lowering agent and an anticancer drug at a cellular level. Growth inhibition through metabolic pathways of metformin is associated with activation of AMP-activated protein kinase (AMPK) via the serine-threonine liver kinase B1 (LKB1), which in turn decreases the phosphorylation of mammalian target of rapamycin (mTOR) and S6 kinase and then results in reducing mRNA translation and protein synthesis [17]. More recent studies have reported on the persisting effects of metformin with several AMPK-independent mechanisms, such as inhibition of IGF-1 receptor signaling through downstream pathways that include extracellular signal-regulated kinase and mTOR, reduction of cAMP level that suppresses protein kinase A activity, or induction of p53-dependent REDD1 expression that leads to mTOR inhibition and cell-cycle arrest in combination with decrease in cyclin D1 expression and pRb phosphorylation and increase in p27 expression. In addition to signaling mechanisms, metformin can also directly scavenge reactive oxygen species (ROS) and block endogenous ROS production [18].
In one case-controlled study, the risk of developing PC among DM patients was reduced with metformin use (odds ratio, 0.38; 95% CI, 0.22 to 0.69; p=0.001), whereas exogenous insulin or an insulin secretagogue actually increased the risk of PC [14]. Another retrospective study of patients with PC (all stages) and DM showed that use of metformin served as a better prognostic index for OS (HR, 0.64; p=0.003). However, the benefit of metformin was not apparent in metastatic PC patients (OS, 8.8 months vs. 7.3 months; p=0.482) [15]. In a recently released study, exposure to metformin had no influence on the prognosis of APC [19].
In our study, 134 patients (73.2% of DM patients) with DM received antidiabetic medication (metformin, 41.8%; sulfonylurea, 45.5%; insulin, 32.1%; other agents, 23.1%). The use of antidiabetic agents as a whole or each of sulfonylurea and insulin showed no association with the prognosis of APC. However, the use of metformin conferred a prognostic benefit on APC patients. The superiority in survival of patients taking metformin was translated to all APC patients as well as within the DM patients (OS, 11.0 months vs. 7.8 months; adjusted HR, 0.697; 95% CI, 0.491 to 1.990; p=0.044) (Fig. 3A), without differences in baseline clinical characteristics (Supplementary Table 3). The metformin-treated DM group had significantly longer survival than the non-DM group or DM without metformin group (p=0.029) (Fig. 3B). Therefore, we concluded that the use of metformin was favorable for prognosis in APC.
Given the difference of primary cancer site, especially the characteristic of pancreas as an organ of insulin secretion, our results have some similarities with those of the recently released analysis of gastric cancer patients who underwent gastrectomy in terms of the decreased mortality rates coupled with increased cumulative use of metformin. In particular, the survival of metformin-treated diabetic patients was not inferior to the survival of non-DM patients in the study [20].
We thought that the recent prospective randomized trials to find the synergism between metformin and chemotherapeutic agents (NCT01210911, NCT01971034, NCT01167738, NCT01666730) could provide us with answers regarding the role of metformin. However, two of them reported that metformin was not beneficial as an add-on therapy to paclitaxel monotherapy or gemcitabine and erlotinib combination therapy. Nevertheless, considering the previous experimental data on the success of metformin with xenograft models, it is still worth waiting for the results of other ongoing trials on metformin in combination with other agents [18].
The prognostic value of duration of DM has rarely been addressed. According to two retrospective studies including resectable PC, one showed shorter OS in recent-onset DM [21], and the other reported increased mortality in long-standing DM (> 5 years) [22]. Recently released meta-analysis from two prospective cohort studies showed decreased survival in long-standing DM (> 4 years) but no difference of survival in recent-onset DM (≤ 4 years), compared with non-DM [23]. In our study, patients with DM tended to survive longer than non-DM patients (HR, 0.788; p=0.059), despite more advanced age (≥ 60 years, p < 0.001). Because there was no difference in survival between the non-DM group and DM without metformin group, we could not exclude the possibility that this advantage of DM patients came from metformin treatment. However, our results showed matched results between the lower HR and extended OS compared to a prior retrospective study which enrolled more LAPC patients than MPC patients and only 6.5% were biguanide users [24]. Some recent studies attempted to interpret PC-related DM with type 3 DM [21], the exogeneous pancreatic failure due to PC, or paraneoplastic syndrome [25] so as to explain better prognosis in DM patients. Due to several limitations in previous studies, including small sample size and small tumor burden, debates regarding the influence of DM on clinical outcomes of APC still remained.
The major limitations of our study originated from the retrospective design. We could not evaluate the duration of antidiabetic medication but we could check whether the patients were taking diabetic medicine during the chemotherapy session. Another drawback is the heterogeneity of antidiabetic medications used. Dual or triple antidiabetic agents, including thiazolidinedione, alpha-glucosidase inhibitor, dipeptidyl peptidase-4 inhibitor, etc., were used simultaneously by more than half the patients. However, the homogeneity of the patient population with advanced disease and good performance status enough for chemotherapy enhances the benefits of our study.
Considering the dearth of research directed at DM and metformin as prognostic factors in APC, we believe that this work represents a significant step forward providing some leverage for patients in addition to palliative chemotherapy.
ConclusionIn conclusion, we have shown that metformin use is a favorable prognostic factor in APC patients receiving palliative chemotherapy. We also showed the tendency that patients with DM survived longer than those without DM. Further studies are warranted to validate the prognostic value of this study, including onset/duration of DM.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
Fig. 1.Overall survival (OS) of all patients by diabetes mellitus (DM) status. Kaplan-Meier estimates, demonstrating positive association between DM and OS (hazard ratio, 0.793; 95% confidence interval, 0.635 to 0.990; p=0.041, log-rank test). mOS, median OS. 
Fig. 2.Overall survival of diabetes mellitus (DM) patients. (A) Recent-onset DM, not having significantly prolonged overall survival (OS) (vs. other DM subsets, including remote-onset DM and subsequent DM; hazard ratio [HR], 0.789; 95% confidence interval [CI], 0.574 to 1.083; p=0.142). (B) OS prolongation in metformin recipients vs non-recipients (HR, 0.693; 95% CI, 0.492 to 0.977; p=0.036). mOS, median OS. 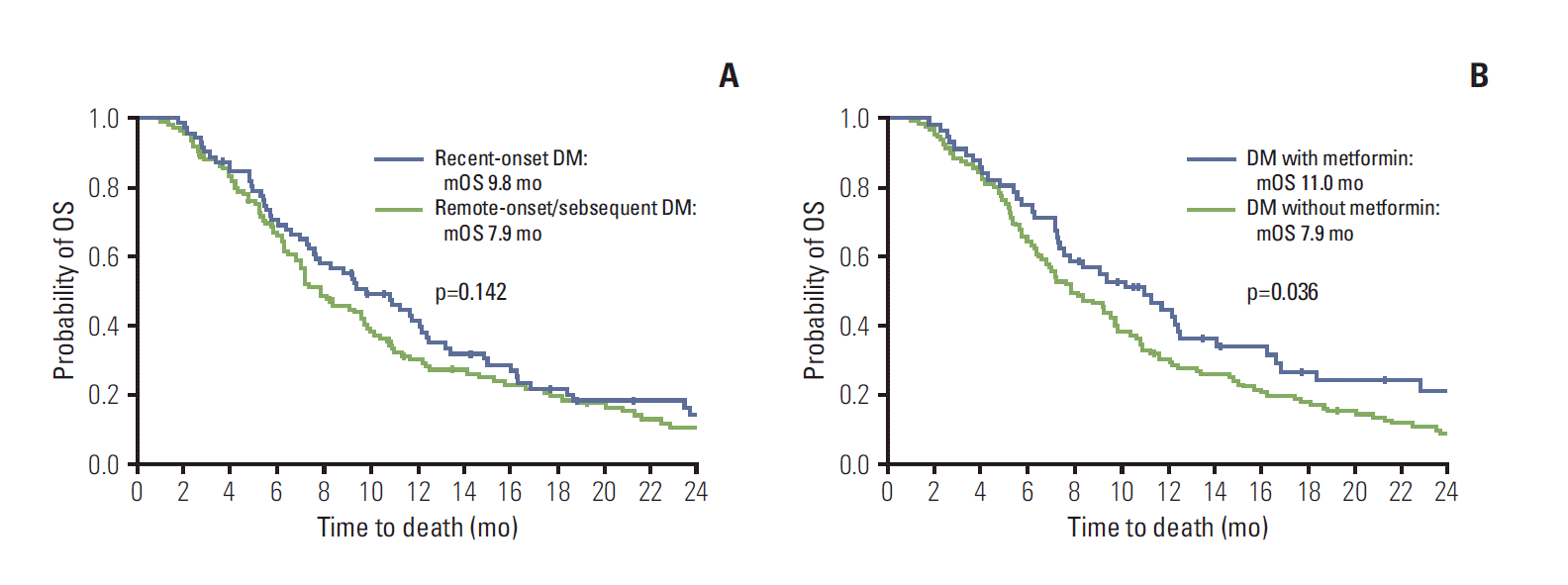
Fig. 3.Overall survival (OS) of all patients by metformin. (A) OS (all patients) by metformin. Prolonged OS in metformin recipients versus non-recipients (hazard ratio [HR], 0.695; 95% confidence interval [CI], 0.509 to 0.948; p=0.022) (adjusted HR with performance, cancer extent, and weight loss [change in body mass index ≥ 1] during first-line therapy, 0.697; 95% CI, 0.491 to 0.990; p=0.044). (B) OS of diabetes mellitus (DM) treated with metformin surpassed that of other groups (non-DM or DM without metformin; p=0.029). 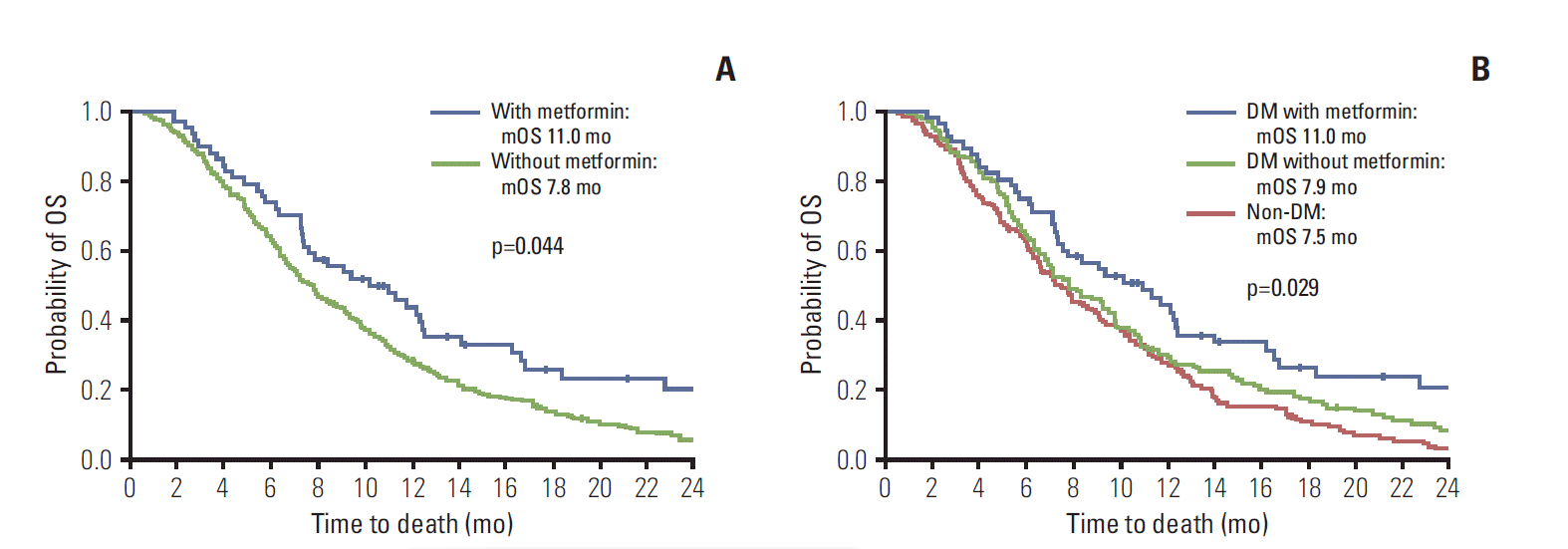
Table 1.Status of DM and antidiabetic medication Table 2.Clinical characteristics by DM status in advanced pancreatic cancer
Table 3.Factors impacting overall survival (all patients)
Table 4.Patient survival by DM subsets
Table 5.Factors impacting overall survival (all patients)
References1. Kris MG, Benowitz SI, Adams S, Diller L, Ganz P, Kahlenberg MS, et al. Clinical cancer advances 2010: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2010;28:5327–47.
2. Andre T, Balosso J, Louvet C, Gligorov J, Callard P, de Gramont A, et al. Adenocarcinoma of the pancreas. General characteristics. Presse Med. 1998;27:533–6.
3. Chiu CC, Huang CC, Chen YC, Chen TJ, Liang Y, Lin SJ, et al. Increased risk of gastrointestinal malignancy in patients with diabetes mellitus and correlations with anti-diabetes drugs: a nationwide population-based study in Taiwan. Intern Med. 2013;52:939–46.
4. Kuuselo R, Savinainen K, Azorsa DO, Basu GD, Karhu R, Tuzmen S, et al. Intersex-like (IXL) is a cell survival regulator in pancreatic cancer with 19q13 amplification. Cancer Res. 2007;67:1943–9.
5. Asano T, Yao Y, Shin S, McCubrey J, Abbruzzese JL, Reddy SA. Insulin receptor substrate is a mediator of phosphoinositide 3-kinase activation in quiescent pancreatic cancer cells. Cancer Res. 2005;65:9164–8.
6. Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–7.
7. Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83.
8. Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95.
9. Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101.
10. Calle EE, Murphy TK, Rodriguez C, Thun MJ, Heath CW Jr. Diabetes mellitus and pancreatic cancer mortality in a prospective cohort of United States adults. Cancer Causes Control. 1998;9:403–10.
11. Wakasugi H, Funakoshi A, Iguchi H. Clinical observations of pancreatic diabetes caused by pancreatic carcinoma, and survival period. Int J Clin Oncol. 2001;6:50–4.
12. Chu CK, Mazo AE, Goodman M, Egnatashvili V, Sarmiento JM, Staley CA, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17:502–13.
13. Mizuno S, Nakai Y, Isayama H, Takahara N, Miyabayashi K, Yamamoto K, et al. Diabetes is a useful diagnostic clue to improve the prognosis of pancreatic cancer. Pancreatology. 2013;13:285–9.
14. Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8.
15. Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905.
16. Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–29.
17. Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73.
18. Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24:469–80.
19. Hwang AL, Haynes K, Hwang WT, Yang YX. Metformin and survival in pancreatic cancer: a retrospective cohort study. Pancreas. 2013;42:1054–9.
20. Lee CK, Jung M, Jung I, Heo SJ, Jeong YH, An JY, et al. Cumulative metformin use and its impact on survival in gastric cancer patients after gastrectomy. Endocr Relat Cancer. 2015 Jan 8 [Epub]. http://dx.doi.org/10.1097/SLA.0000000000001086.
22. Hwang A, Narayan V, Yang YX. Type 2 diabetes mellitus and survival in pancreatic adenocarcinoma: a retrospective cohort study. Cancer. 2013;119:404–10.
23. Yuan C, Rubinson DA, Qian ZR, Wu C, Kraft P, Bao Y, et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. J Clin Oncol. 2015;33:29–35.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||