Introduction
Polycomb group proteins are initially identified as regulators controlling the establishment of body segmentation by silencing hox genes expression in Drosophila. Later, they were foun to be epigenetic regulators that are critical for multiple cellular functions, including stem cell maintenance and differentiation [1]. Polycomb group proteins are well conserved between Drosophila and humans and are involved in gene silencing. Two major polycomb repressive complexes, Polycomb repressive complex (PRC) 1 and PRC2, control gene silencing through post-translational modifications of histone proteins [2]. PRC1 consists of Bmi1, Ring1b, CBX4, and PHC subunits and induces histone 2A lysine 119 ubiquitination (H2AK119ub1). In contrast, PRC2 consists primarily of enhancer of zeste homolog 2 (EZH2), EED, SUZ12, and RbAp48 and catalyzes the methylation of histone H3 lysine 27 (H3K27) to generate trimethyl-H3K27 (H3K27me3) [3]. PRC1 enhances the effects of PRC2 by recognizing H3K27me3 and interacting with it, but these complexes can also repress gene expression independently [2]. EZH2 is the catalytic subunit of PRC2, and growing evidence demonstrates that EZH2 is essential for cancer initiation, development, progression, metastasis, and drug resistance. Therefore, EZH2 is currently considered a promising drug target, and multiple inhibitors of EZH2 have been developed, some of which are in clinical trials. In this review, we introduce current information regarding the molecular mechanisms by which EZH2 expression/activity is regulated as well as the role of EZH2 in oncogenic signaling pathways. Moreover, we focus on the therapeutic potential of EZH2 and discuss possible approaches to targeting EZH2.
Regulation of EZH2 Expression and Activity in Cancer
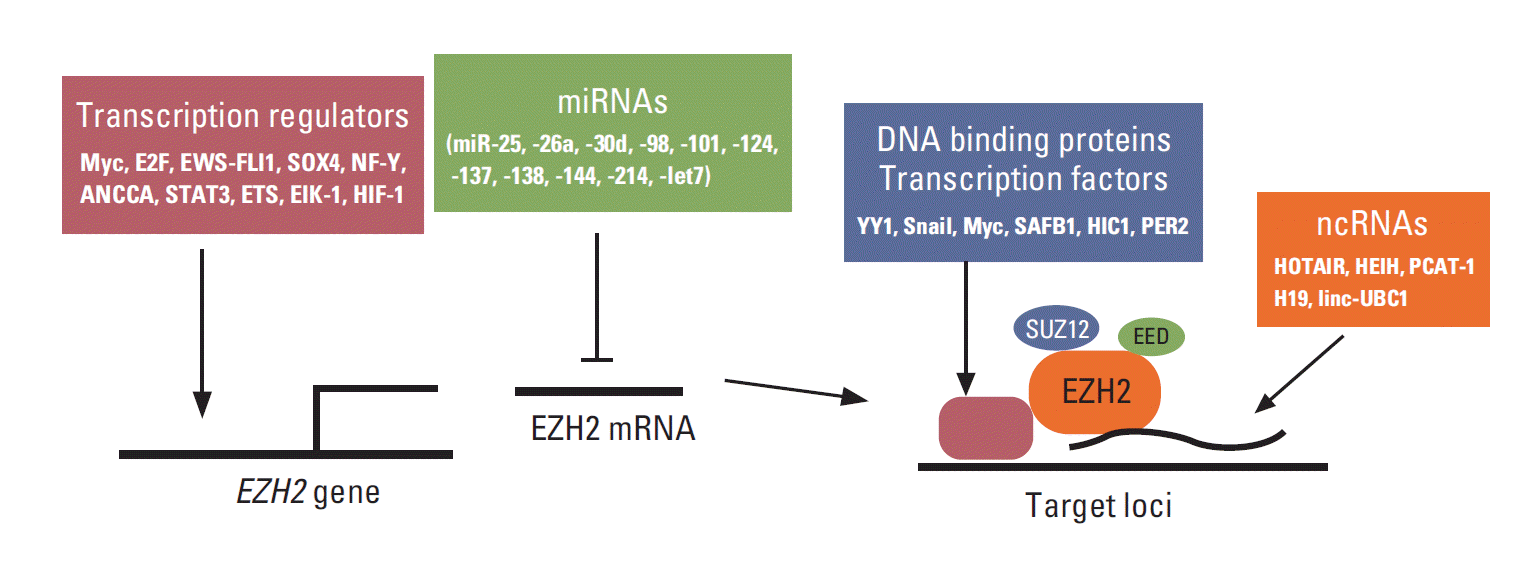
EZH2 is frequently overexpressed in many cancer types and is critical for cancer cell proliferation and survival. Indeed, the regulators of EZH2 expression are also critical for cell proliferation, tumorigenesis, and stem cell maintenance (Fig. 1). For example, Myc binds to EZH2 promoter and directly activates its transcription, and EZH2 expression is correlated with Myc expression in prostate cancer [4]. Myc also upregulates EZH2 expression by downregulating miRNA 101 (miR-101), miR-26a, and miR-26b [4-7]. In contrast, c-Myc expression is also positively regulated by EZH2 in glioblastoma, although the underlying mechanism is uncertain [8]. In addition to Myc, another cell cycle regulator, E2F, positively controls EZH2 transcription, and EZH2 is critical for the regulation of pRB-E2F pathway [9]. ANCCA, a co-activator of androgen receptor (AR) and binding protein of E2F, can enhance E2F-mediated EZH2 transcription in prostate cancer cells [10,11]. In Ewing tumors, EWS-FLI1 fusion oncoprotein directly regulates EZH2 gene expression [12]. SOX4, one of the key regulators of stem cells, directly regulates the expression of EZH2 mRNA, which is critical for SOX4-mediated epithelial-mesenchymal transition (EMT) [13]. Moreover, NF-Y, STAT3, and ETS transcription factors directly regulate EZH2 transcription in epithelial ovarian, colorectal, and prostate cancer cells, respectively [14-16]. Both Elk-1 and HIF1 directly regulates EZH2 transcription that is associated with aggressive breast cancer [17,18].
In addition to transcriptional regulators, multiple miRNAs have been shown to directly regulate EZH2 expression, and many of them are deregulated in cancer. So far, miR-25, -26a, -30d, -98, -101, -124, -137, -138, -144, -214, -let-7, and -let-7a have been shown to be able to downregulate EZH2 expression directly in cancer cells. The downregulation of these miRNAs and the resulting upregulation of EZH2 seem to be critical for the aggressive behaviors of various cancers. These miRNAs include miR-25 and -30d in thyroid cancer [19]; miR-26a in lymphoma, nasopharyngeal carcinoma (NPC), and breast and prostate cancer [6,7,20,21]; mR-101 in NPC, glioblastoma multiforme (GBM), and prostate, bladder, gastric, head and neck (HN), and non-small cell lung cancer (NSCLC) [22-27]; miR-138 in HN cancer, GBM, and NSCLC [28-30]; let-7s in prostate cancer and NPC [31,32]; miR-124 in hepatocellular carcinoma (HCC) and gastric cancer [33,34]; miR-98 in NPC and gastric cancer [35,36]; miR-137 in melanoma [37]; miR-144 in bladder cancer [38]; and miR214 in gastric cancer and HCC [35,39]. These miRNAs are tumor suppressor like miRNA and, interestingly, miR-26a has been also shown to be regulated by epidermal growth factor receptor-mediated Ago2 phosphorylation under hypoxia condition [40].
Interaction Partners That Regulate the Recruitment of PRC2 to Specific Loci
EZH2, EED, SUZ12, and RbAp48 are core proteins in PRC2, but their DNA binding activity is weak. Thus, PRC2 requires other factors to recruit it to specific loci. Multiple transcription factors also interact with PRC2 to recruit it to specific loci, and some of them have been shown to play a critical role in cancer. Transcription factor Yin Yang 1 (YY1) interacts with EZH2 and recruits it to the specific sites to regulate gene silencing. YY1 and PRC2 are involved in muscle differentiation [41]. In endometrioid endometrial carcinoma, EZH2 and YY1 repress tumor suppressor APC and promote cell growth [42]. Snail forms a complex with EZH2 via histone deacetylase (HDAC)1/2 and recruits it to E-cadherin promoter to suppress E-cadherin expression in NPC [43]. c-Myc interacts with EZH2 and suppresses miR-101 expression in HCC, whereas MYCN interacts with EZH2 and inhibits tumor suppressor clusterin in neuroblastoma [5,44]. In addition to oncogenic transcription factor, PRC2 interacts with tumor suppressor proteins and contributes to tumor suppressor function. For example, tumor suppressor scaffold attachment factor B1 (SAFB1) interacts with PRC2 and AR and represses AR transcription machinery via H3K27me3 in prostate cancer cells [45]. Hypermethylated in cancer 1 (HIC1), which is a tumor suppressor gene that is frequently silenced or deleted in various cancers, recruits PRC2 to its target genes [46]. PER2 can interact with PRC2 and Oct1, and recruit them to Snail Slug and Twist promoters and inhibit their gene expression, thereby using PRC2 as a tumor suppressor [47]. Other transcription factors such as E2F6, Twist-1, RUNX3, and CCCTC binding factor interact with PRC2 and recruit it to repress specific target genes, but their roles in cancer are uncertain [48-51].
In addition to proteins, noncoding RNAs (ncRNAs) interact with EZH2 and play an important role in the recruitment of EZH2 to several specific loci. In cancer, HOTAIR is one of the most well described large intervening ncRNAs that interacts with EZH2 [52]. Overexpression of HOTAIR in breast cancer cells enhances cancer cell invasion and metastasis that require PRC2, while the loss of HOTAIR reduces them. HOTAIR plays an oncogenic role in colorectal cancer, pancreatic cancer, and NSCLC [53-55]. Remarkably, HOTAIR can interact with PRC2 and the LSD1/CoREST/REST repressor complex (which is responsible for the demethylation of H3K4me2), serving as a scaffold to recruit two distinct histone modifiers to the same loci [56].
In addition to HOTAIR, several ncRNAs have been shown to interact with EZH2 and are involved in EZH2-mediated cancer aggressiveness. These include HEIH in HCC [57], PCAT-1 in prostate cancer [58], and H19 and linc-UBC1 in bladder cancer [59,60]. Several other ncRNAs such as Xist, Six3OS, Meg3, AS1DHRS4, and ANCR have been shown to interact with PRC2 and regulate X-chromosome inactivation, cell differentiation, and stem cell maintenance [61-65], but their roles in cancer have not been identified. In addition to ncRNA, miR-320 directly interacts with EZH2 and argonaute-1 (AGO1) and recruits them to the promoter region of the cell cycle gene POLR3D and silences it [66]. Moreover, EZH2 also interacts with multiple intronic RNAs. Among them, the intronic RNA for SMYD3 (H3K4 methyltransferase) reduces SMYD3 expression, cell proliferation, and xenograft tumor growth in human colorectal cancer cells [67]. Interestingly, BRCA1 negatively regulates PRC2 activity by inhibiting the association between EZH2 and HOTAIR, and the loss of BRCA1 contributes to an aggressive breast cancer phenotype [68]. EZH2-HOTAIR or EZH2-Xist interaction is also regulated by CDK-mediated phosphorylation, as described in the next section [69]. PRC2 co-factor JARID2 also mediates the interaction of PRC2 and ncRNAs such as Xist and Meg3 [65,70].
Post-translational Modification of EZH2
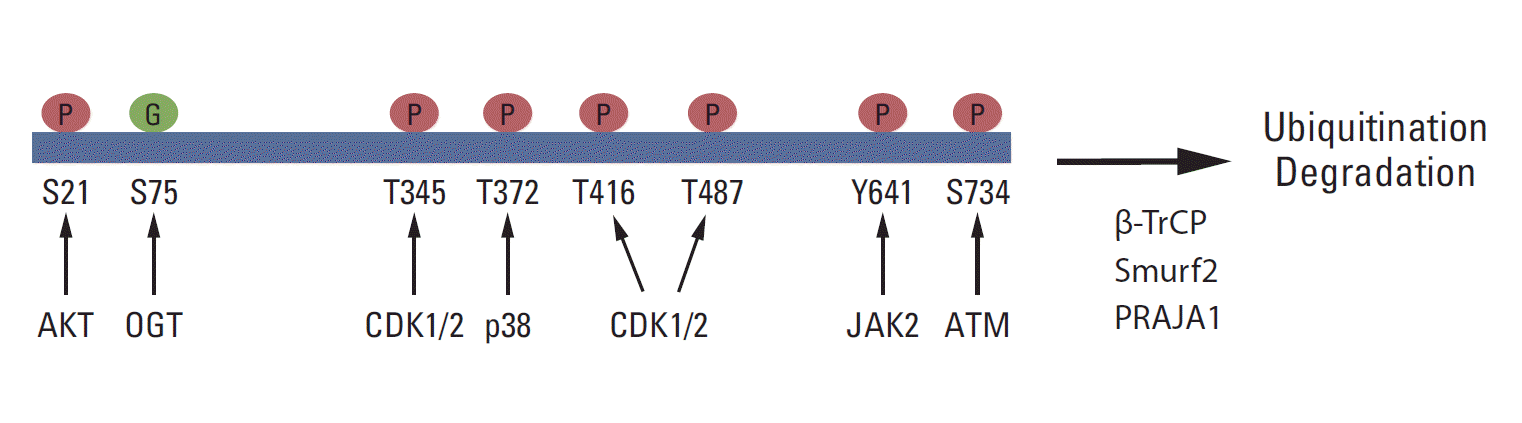
Growing evidence shows that EZH2 activity and stability are regulated by post-translational modifications and that these modifications are critical for the biological function of PRC2 (Fig. 2). It has been reported that Akt phosphorylates EZH2 at serine 21 (S21) and inhibits its enzyme activity for H3K27me3 [71]. Later, this phosphorylation site was shown to be critical for the H3K27me3-independent function of EZH2 [72,73]. JAK2 phosphorylates EZH2 at tyrosine 641 (Y641), which promotes the interaction of EZH2 with β-TrCP and degradation of EZH2 [74]. Y641 is frequently mutated in B-cell lymphoma, and the stability and activity of the EZH2 Y641 mutant is higher than that of wild-type EZH2. Several studies have demonstrated that CDK1/2 phosphorylates EZH2 at multiple sites, including threonine 345, T416, and T487 [69,75-78]. The role of CDK-mediated phosphorylation in EZH2 function is diverse and may depend on cell types and conditions. T345 phosphorylation promotes the association between EZH2 and HOTAIR, whereas T416 phosphorylation induces the binding of NIPP1 to EZH2, and both T345 and T416 phosphorylation are critical for the recruitment of EZH2 to specific loci [69,78]. Moreover, NIPP1 maintains EZH2 phosphorylation by inhibiting its dephosphorylation by PP1 [78]. It has been showed that CDK1 phosphorylates EZH2 at T487 and that the phosphorylation induces the dissociation of EZH2 from PRC2, resulting in the inactivation of EZH2 and a reduction in cancer cell invasion [77]. In contrast, EZH2 phosphorylation at T345 promotes cell migration and proliferation [75]. T345 and T487 phosphorylation in EZH2 also promotes EZH2 ubiquitination and degradation [76].
In neurons, ATM interacts with and phosphorylates EZH2 at S734, and S734 phosphorylation of EZH2 reduces PRC2 assembly, EZH2 stability, and cell death in neurons [79]. ATM-mediated phosphorylation of EZH2 is critical for neurodegeneration in ataxia-telangiectasia, which is caused by ATMmutation [79]. Moreover, p38 phosphorylates EZH2 at threonine 372 (T372) and promotes its interaction with YY1, which is critical for tumor necrosis factor-mediated Pax7 inhibition and muscle stem cell proliferation [80]. The role of ATM- or p38-mediated phosphorylation in cancer is not yet certain.
Recently, EZH2 was shown to interact with O-linked N-acetylglucosamine (GlcNAc) transferase (OGT) and to be O-GlcNAcylated at S75 in vivo. Interestingly, OGT upregulates cellular H3K27me3 levels, and S75 to alanine (S75A) mutant EZH2 is less stable than wild-type EZH2, suggesting that the O-GlcNAcylation of EZH2 may play a role in EZH2 stability and H3K27me3 [81]. EZH2 is also sumoylated in vivo and in vivo, but the functional significance of its sumoylation has not been determined [82]. EZH2 ubiquitination is critical for its protein stability. It has been shown that Smurf2 functions as an E3 ligase for EZH2 in human mesenchymal stem cells and promotes neuron differentiation [83]. β-TrCP and PRAJA1 also function as E3 ligases for EZH2 [74,84].
Function of EZH2 in Cancer
EZH2 is required for cancer cell proliferation, migration, invasion, and EMT, all of which are associated with cancer initiation, progression, and metastasis. More importantly, EZH2 is closely associated with stem cell properties, especially cancer stem cell properties, and tumor-initiating cell function [8,17,85].
In diffuse large B-cell lymphoma and follicular lymphoma, recurrent somatic mutations in the EZH2 gene have been identified, which changes amino acid tyrosine 641 (Y641) in EZH2, thereby altering its enzyme activity [86]. These mutations were originally considered a loss-of-function mutation because it reduces EZH2 methyltransferase activity toward an unmodified substrate. However, mono- to di- and di- to trimethylation activity is higher in Y641 mutant EZH2 than in wild-type EZH2. Y641 mutant EZH2 actually has higher activity of mono- to di- and di- to tri-methylation than wildtype EZH2 [87]. In addition, the Y641 mutation is always a heterogeneous mutation, and diffuse large B-cell lymphoma and follicular lymphoma with EZH2 mutation express both wild-type and Y641 mutant EZH2, resulting in higher H3K27me3 in mutant cancer cells than wild-type cells [87]. Thus, the EZH2 Y641 mutation is unique gain-of-function mutation. The oncogenic role of the Y641 mutation was further confirmed in an engineered mouse model in which conditional expression of mutant EZH2 in germinal center B-cells induced germinal center hyperplasia and promoted lymphoma formation in the presence of Bcl-2 overepxression [88]. In addition to Y641 mutation, A687V and A677G mutations have been identified as activating mutations of EZH2 in B-cell lymphoma [89,90]
Recently, a K27M mutation in one of the histone H3 variants, H3.3, was found in 50% of pediatric high-grade glioma [91,92]. The cells with H3.3K27M show reduced levels of global H3K27me3 because H3.3K27M binds to and inhibits EZH2. Interestingly, H3K27me3 and EZH2 were also shown to be locally increased in hundreds of genes in cells with the H3.3K27M mutation [93]. Therefore, alterations in H3K27me3 are closely associated with glioma.
Overexpression of EZH2 is frequently observed in multiple cancer types, including prostate, breast, bladder, ovarian, lung, liver, brain, kidney, gastric, esophageal, and pancreatic cancer and melanoma [94-104]. In many of these, EZH2 expression is also correlated with higher proliferation and aggressive behavior of cancer cells as well as poor prognosis. Indeed, multiple studies have shown that overexpression of EZH2 promotes cell proliferation, migration, and/or invasion in vivo [26,43,100,105]. Furthermore, overexpression of wild-type EZH2 in mammary epithelial cells in vivo results in epithelial hyperplasia and promotes mammary tumor initiation induced by human epidermal growth factor receptor 2/neu expression [106,107].
In some types of cancer, EZH2 functions as a tumor suppressor. Inactivating mutations of EZH2 are found in patients with myeloid malignancies including myelodysplastic syndrome and myeloproliferative neoplasms, and such EZH2 mutations are associated with poor patient survival [108,109]. Mice with conditional deletions of EZH2 and TET2 in hematopoietic stem cells, the mutations of which frequently co-exist in myeloid malignancies, develop myelodysplastic syndrome and myeloproliferative neoplasms [110]. In addition to myeloid malignacies, 25% of T-cell leukemia cases have been shown to have loss-of-function mutations and deletions of the EZH2 and SUZ12 genes [111]. Indeed, conditional deletion of EZH2 in bone marrow cells causes T-cell leukemia, indicating that EZH2 functions as a tumor suppressor in T-cell leukemia as well [112]. Moreover, mice with conditional deletion of EZH2 in the pancreatic epithelium also exhibit impaired pancreatic regeneration and acceleration of K-Ras-induced neoplasia [113]. Together, these results suggest that the role of EZH2 is cell context dependent, although EZH2 functions as an oncogenic factor in the majority of solid tumors.
EZH2 Targets in Cancer
So far, many EZH2 target genes have been identified, and HOX genes are well-known targets for EZH2 during embryonic development. Because EZH2 frequently functions as an oncogenic factor in many cancer types, most EZH2 targets identified in cancer are tumor suppressor genes. The INK4B-ARF-INK4A tumor suppressor locus is regulated by EZH2, PRC1, and PRC2, and the suppression of these genes is also critical for development of embryo as well as cancer [114-117]. Another critical target of EZH2 in multiple cancers is the E-cadherin gene (CHD1), the downregulation of which is critical for EMT and metastasis [118-121]. EZH2 also interacts with Snail to repress E-cadherin expression [43].
In addition to these proteins, multiple EZH2 target genes have been shown to be involved in EZH2-mediated cancer aggressiveness. These target genes include stathmin and Wnt antagonists in HCC [122,123]; bone morphogenetic protein receptor 1B in GBM [85]; p57 in breast and ovarian cancers [124,125]; DAB2IP, SLIT2, TIMP2/3, and CCN3/NOV in prostate cancer [126-129]; FOXC1, HOXC10, and RAD51 in breast cancer [130-132]; CXXC4 in gastric cancer [133]; MyoD in rhabdomyosarcoma [134]; rap1GAP in HN cancer [25]; CASZ1 in neuroblastoma [135]; and RUNX3 and KLF2 in multiple cancer types [136,137]. In addition, several molecules such as Bim, TRAIL, and FBXO32 play a role in apoptosis induced by the inhibition of EZH2 [138-140]. Vasohibin1 is regulated by EZH2 in tumor-associated endothelial cells, and this regulation plays a role in tumor angiogenesis [141]. EZH2 also regulates the expression other epigenetic regulators by silencing multiple miRNAs, which are critical for the oncogenic function of EZH2 [142,143].
H3K27me3-Independent Functions of EZH2
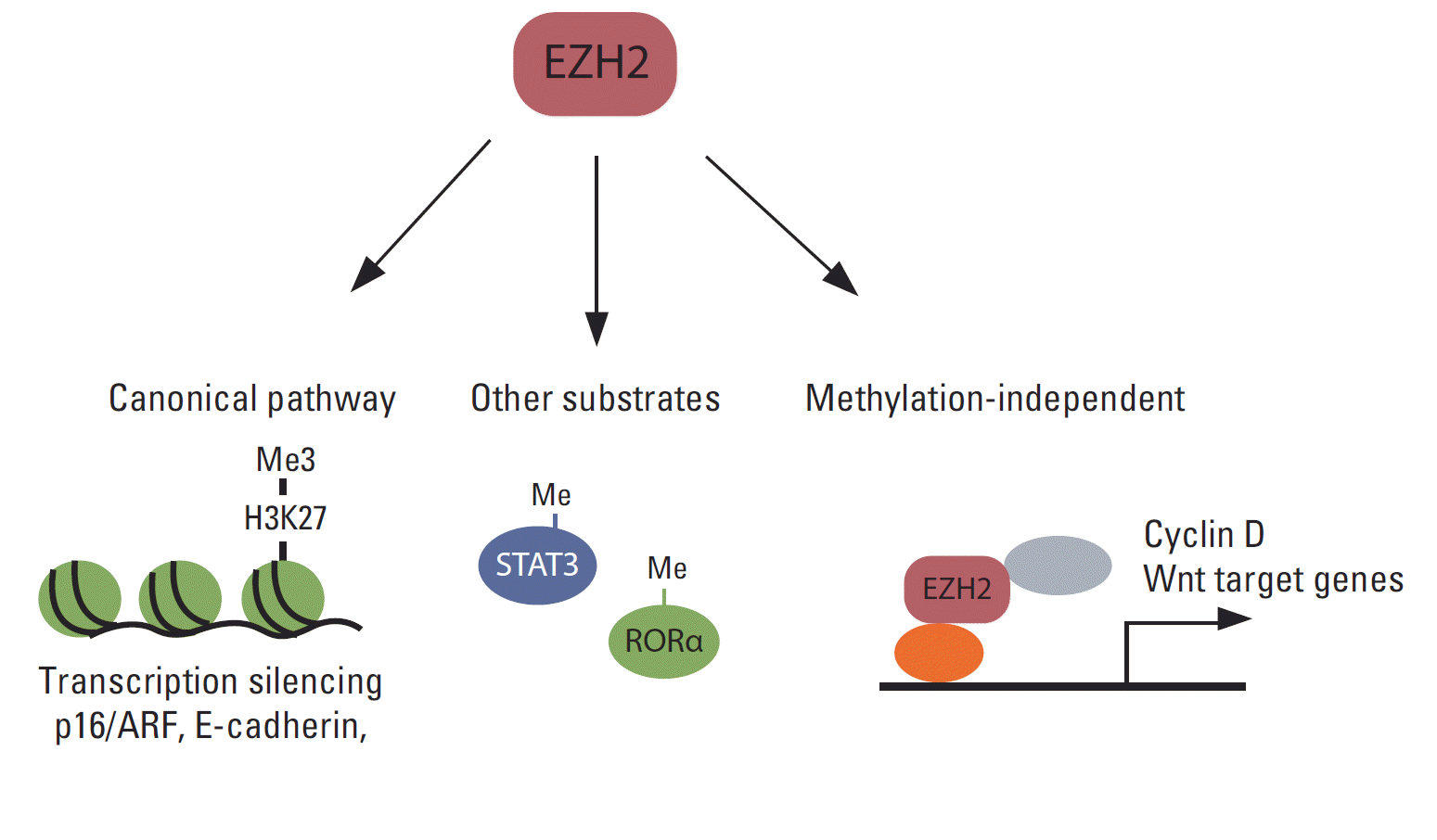
Although the primary function of EZH2 is gene silencing through the methylation of H3K27, accumulating evidence shows that EZH2 functions independently of H3K27me3 in various cancers (Fig. 3). Several reports have shown that EZH2 functions as a transcription activator. For example, EZH2 interacts with estrogen receptor (ER) α and β-catenin, and the complex regulates c-Myc and cyclin D1 expression in breast cancer cells [144]. Moreover, in a transgenic mouse model, EZH2 was shown to interact with β-catenin and promote its nuclear accumulation and activation in mammary epithelial cells [107]. In colon cancer cells, the DNA repair protein proliferating cell nuclear antigen (PCNA)-associated factor interacts with EZH2 and β-catenin and increases β-catenin target gene expression [145]. The effect of EZH2 on PCNA-associated factor-mediated activation of β-catenin does not require EZH2 methyltransferase activity. EZH2 also functions as a transcriptional co-activator with AR in castration-resistant prostate cancer cells [72]. Interestingly, this functional switch from a transcription silencer to an activator requires S21 phosphorylation of EZH2 by Akt, and activation of AR depends on EZH2 methyltransferase activity. In ER-negative basal-like breast cancer cells, EZH2 interacts with RelA/RelB and functions as a transcription co-activator of nuclear factor-kappa B. In contrast, EZH2 interacts with ER and represses nuclear factor-kappa B target gene expression by inducing H3K27me3 on their promoters in ER-positive luminal-like breast cancer cells [146]. In natural killer/T-cell lymphoma, EZH2, which is upregulated via Myc-mediated miRNA inhibition, directly activates cyclin D transcription and promotes cell proliferation independent of methyltransferase activity [147].
EZH2 also methylates proteins other than histone H3 and modulates their functions (Fig. 3). For example, EZH2 interacts with and methylates STAT3, resulting in increased tyrosine phosphorylation and activation of STAT3 [73]. Strikingly, AKT-mediated phosphorylation at S21 in EZH2 is critical for the interaction of EZH2 with STAT3, and this AKT-EZH2-STAT3 pathway is critical for the maintenance of glioblastoma stem cells and tumor progression. EZH2 also mono-methylates tumor suppressor, retinoic acid-related orphan nuclear receptor α (RORα) [148]. Mono-methylated RORα is recognized by the DCAF1/DDB1/CUL4 E3 ubiquitin ligase complex and undergoes ubiquitination and degradation. EZH2 also methylates GATA4 and inhibits its activity by inhibiting its interaction with p300 [149], although the role of this methylation in cancer has not been established.
EZH2 also regulates cellular functions other than transcription. Cytosolic EZH2, the level of which is higher in prostate cancer cells than in normal prostate cells, regulates actin polymerization [150,151]. However, the underlying molecular mechanism has not yet been identified. In addition, PRC2 is recruited to sites of DNA damage in a poly(ADP-ribose) polymerase-dependent manner and is involved in DNA damage repair [152]. EZH2 knockdown reduces DNA double-strand break repair and sensitizes cells to ionizing radiation. Interestingly, EZH2 and BRCA1 regulate each other and are involved in several cellular functions. Knockdown of EZH2 upregulates BRCA1 protein, which is important for the downregulation of proliferation induced by the inhibition of EZH2 in ER-negative breast cancer [153]. Consistently, EZH2 induces BRCA1 nuclear exclusion and inhibits its activity, which contributes to chromosome instability in breast cancer [154]. In contrast, BRCA1 also regulates EZH2 activity. BRCA1 inhibits EZH2-HOTAIR interaction as previously described herein [68]. Moreover BRCA1-deficient cells have higher EZH2 expression and are thereby more sensitive to EZH2 inhibition than BRCA1-proficient cells [155]. Thus, EZH2-BRCA1 interaction is complicated, and further studies may be necessary.
Therapeutic Implications of EZH2
Because EZH2 is a central regulator of proliferation, migration, invasion and stem cell properties of cancer cells, it is considered a potential drug target. 3-Deazaneplanocin A (DZNep), which is an inhibitor of S-adenosylhomocysteine hydrolase, downregulates PRC2 proteins including EZH2 and inhibits PRC2 activity [139]. DZNep treatment indeed induces the downregulation of H3K27me3, reactivates PRC2 target genes, and effectively induces apoptosis in cancer cells but not in normal cells [139]. This compound has been widely used in preclinical and in vitro studies to investigate the function of EZH2 in cancer and has been shown to effectively inhibit cell proliferation and tumor growth in various cancers [156-159]. Remarkably, the killing effect of DZNep is about 20-fold greater in BRCA1-deficient cells than in BRCA1-proficient mammary tumor cells although the underlying mechanism is not known [155]. DZNep was recently shown to induce erythroid differentiation independent of EZH2, suggesting that the effects of DZNep may be partially independent of EZH2 inhibition [160]. However, because DZNep downregulates EZH2 protein levels, it is expected to inhibit the methylation-independent functions of EZH2 [147].
Recently, several highly selective small molecule inhibitors against EZH2, such as GSK126, EPZ005687, EI1, and EPZ-6438, have been developed [161-164]. These inhibitors exhibit higher effects against the lymphoma with Y641 activation mutation of EZH2 than the one with wild-type EZH2. Currently, EPZ-6438 is being tested in clinical trials of patients with B-cell lymphoma and advanced solid tumors.
In addition to specific EZH2 inhibitors, several other drugs and compounds have been reported to be able to downregulate EZH2, and the downregulation of EZH2 is critical for their anti-cancer activity. These include curcumin [165,166], omega-3 polyunsaturated fatty acids [167], and sorafenib [168]. Moreover, inhibition of EZH2 also sensitizes cancer cells to various other anti-cancer drugs, such as HDAC inhibitors, imatinib, gemcitabine, paclitaxel, and cisplatin [27,98,140,169-174].
Conclusion
EZH2 is a critical regulator of cell proliferation, migration/invasion, and stemness in cancer and functions as an oncogenic factor in most solid tumors. Indeed, EZH2 inhibitors have shown promising anti-cancer activity against EZH2-active or -overexpressing cancer cells in multiple preclinical studies, and EPZ-6438 is currently under clinical trials. Inhibition of EZH2 also enhances several existing anticancer drugs, suggesting the potential for combination therapy using EZH2 inhibitors. Moreover, EZH2 is frequently overexpressed in multiple cancer types and is associated with poor prognosis. Therefore, EZH2 may serve as a valuable prognostic marker. In the future, additional studies will be required to establish effective combination treatment strategies and identify appropriate biomarkers in various cancer types to predict sensitivity to EZH2 inhibitors.
Herein, we introduced multiple mechanisms of EZH2 regulation, including transcriptional regulation, mRNA regulation by miRNAs, accessibility to DNA via DNA binding proteins and ncRNAs, and post-translational modifications. Because these upstream regulators of EZH2 most likely control multiple targets other than EZH2, the inhibition of these mechanisms may be an alternative approach to targeting EZH2 and even more effective than EZH2 inhibitors alone. For instance, the kinases that phosphorylate EZH2 also phosphorylate many substrates and activate other signaling pathways. Indeed, CDK inhibitors have shown anti-tumor activity in preclinical studies and are currently being tested in clinical trials. The effects of CDK inhibitors may be achieved partially through the attenuation of EZH2 activity, and EZH2 may serve as a biomarker for these drugs. Thus, the identification of upstream regulators of EZH2 may lead to effective therapeutic strategies for various cancers.