How Molecular Understanding Affects to Prescribing Patterns and Clinical Outcome of Gefitinib in Non-small Cell Lung Cancer? 10 Year Experience of Single Institution
Article information
Abstract
Purpose
Gefitinib was introduced in 2002 for treatment of non-small cell lung cancer (NSCLC); however, it is not clear whether its use in daily practice has changed the outcome of patients. The purpose of this study is to evaluate the question of how molecular understanding regarding gefitinib and epidermal growth factor receptor (EGFR) mutation affect the prescribing patterns and clinical outcomes of treatment with gefitinib in NSCLC, in a real practical field.
Materials and Methods
We conducted a retrospective analysis of the consecutive database of NSCLC patients who were treated with gefitinib at Seoul National University Hospital between January 2002 and December 2011. Prescribing patterns and clinical outcomes were analyzed by year.
Results
A total of 1,115 NSCLC patients, who received gefitinib at recurred or metastatic setting, were included in this study. Proportion of patients receiving gefitinib, for the first line, showed a gradual increase, from 5.2% in 2002-2003 to 30.6% in 2010-2011. Proportion of patients who underwent EGFR mutation testing showed a rapid increase, from 0.6% in 2004-2005 to 73.5% in 2010-2011. The response rate also showed a gradual increase, from 17.2% in 2002-2003 to 57.1% in 2010-2011 (p<0.001). The median progression-free survival of gefitinib was increased with statistical significance from 2.8 months in 2002-2003 to 9.1 months in 2010-2011 (p<0.001).
Conclusion
We demonstrated that molecular understanding and practical use of EGFR mutation testing have resulted in a change in the prescription patterns of gefitinib. Use of an enrichment strategy can lead to improvement in the efficacy of gefitinib in real practice.
Introduction
Gefitinib is an orally active, selective epidermal growth factor receptor (EGFR), tyrosine kinase inhibitor (TKI), which blocks the signal transduction pathway implicated in proliferation and survival of cancer cells. The results of phase I trials were reported in 2002. Of particular interest, major tumor regression and prolonged duration of response were observed with gefitinib in some patients with heavily treated non-small cell lung cancer (NSCLC) [1-4]. This inspiring result subsequently led to conduct of two phase II trials in NSCLC [5,6], and antitumor activity was demonstrated with good tolerability. Following the results of these two phase II studies [5,6], gefitinib was approved for treatment of patients with advanced NSCLC in Japan in July 2002, in the US in May 2003, and in Korea in June 2003.
At that time, researchers were aware that the response rate to gefitinib was higher among women, patients with adenocarcinoma, never-smokers, and East Asians [5-7], although they did not know the exact reason. Then, in April 2004, somatic mutations in the kinase domain of EGFR, mainly in-frame deletions in exon 19, and a missense mutation in exon 21, were suggested to be the determinants of gefitinib sensitivity [8,9]. Strong correlation of EGFR mutation and clinical outcomes in NSCLC patients treated with gefitinib was consistently confirmed in retrospective studies [10-12]. It was also reported that EGFR mutations are common in females, never-smokers, patients with adenocarcinoma, and Asians [13], which explains why these patients were sensitive to gefitinib. These findings were also repeatedly confirmed in a prospective phase III trial [14-21], and gefitinib has been known to provide a survival benefit to NSCLC patients with EGFR mutation.
With deepening of molecular knowledge regarding gefitinib and EGFR mutation derived from the bench and pivotal clinical studies [14-21], clinicians are able to optimize the use of gefitinib more judiciously. More knowledge regarding molecular targeted agents allows clinicians to select patients who are likely respond to and benefit from gefitinib, before the actual use of gefitinib. However, little data are available concerning how this molecular understanding regarding gefitinib affects the prescribing patterns, and real treatment outcome. It is not yet certain whether knowledge of molecular targeted agents can lead to real improvement of treatment outcomes outside the clinical trials.
The purpose of this study is to evaluate the question of how molecular understanding regarding gefitinib and EGFR mutation affects the prescribing patterns and clinical outcome of gefitinib in NSCLC in the real practical field.
Materials and Methods
1. Study patients and gefitinib treatment
We conducted a retrospective analysis of the consecutive database of NSCLC patients who were treated with gefitinib at Seoul National University Hospital between January 2002 and December 2011. We reviewed the medical records of patients and clinical parameters were reviewed. Never-smoker was defined as ≤100 cigarettes in a lifetime. A total of 1,115 patients were analyzed. All patients had pathologically proven recurred or metastatic NSCLC. Gefitinib was taken orally at the dose of 250 mg daily until tumor progression, death, significant uncontrolled toxicity, or patient refusal. Patients were re-evaluated every three or four weeks by a chest X-ray or computed tomography, and adequate blood tests for tumor response and toxicity. Treatment response was evaluated based on the Response Evaluation Criteria in Solid Tumors criteria. Patients with complete response or partial response were regarded as responders. Clinical outcomes and prescribing patterns were compared by year.
2. Mutation analysis
For analysis of EGFR mutation, we used paraffin-embedded tissue of the primary tumor samples. Mutation analysis of EGFR exons 18, 19, 20, and 21 was performed as previously described [22]. In brief, genomic DNA from tumors was extracted from five 5-µm paraffin sections containing a representative portion of each tumor block, using QIAamp DNA Mini kits (Qiagen, Hilden, Germany). Coding sequences from exons 18 to 21 were amplified using polymerase chain reaction (PCR), with both forward and reverse sequence-specific primers [22]. PCR fragments were sequenced and analyzed in both sense and antisense directions. All sequence variants were confirmed by sequencing the products of independent PCR amplifications in both directions. Sequence data were generated using the ABI PRISM 3730 DNA Analyzer (Applied Biosystems, Foster City, CA). Sequences were analyzed using Sequencer software (Applied Biosystems) for comparison of variations.
3. Statistical analysis
Statistical analyses of the categorical variables were performed using Pearson's χ2 test. Progression-free survival (PFS) of gefitinib was determined as the interval between gefitinib and the date when disease progression or death was first documented. Overall survival (OS) was measured from the date of initiation of gefitinib until the date of death, or the last follow-up visit. The median duration of PFS and OS was calculated using the Kaplan-Meier method. Comparisons of survival between the different groups were performed using the log-rank test. A two-sided p<0.05 was considered statistically significant. All analyses were performed using SPSS ver. 19.0 (IBM Corp., Armonk, NY). The study was reviewed and approved by the Institutional Review Board of Seoul National University Hospital, and was conducted in accordance with the Principles of the Declaration of Helsinki.
Results
1. Patients and clinical outcomes
A total of 1,115 NSCLC patients who received gefitinib at the recurred or metastatic setting were analyzed in this study. The median age was 61 years (range, 25 to 91 years). A summary of the characteristics of patients and tumors is shown in Table 1. The overall response rate was 35.4% (95% confidence interval, 32.2 to 37.8%). The median PFS and OS were 4.5 months and 24.4 months, respectively.
2. Prescribing patterns and EGFR mutation test by year
Prescribing patterns have changed and lines of TKI chemotherapy have been moved upfront, toward the first line (Table 2). In the year 2002-2003, 41.4% of patients received gefitinib, as their fourth line or more. However, with passage of time, the portion of patients receiving gefitinib as a fourth line or more showed a steady decrease, and only 4.9% of patients received it as a fourth line or more in the year 2010-2011 (p<0.001). In addition, the proportion of patients receiving gefitinib, as a first line, showed a gradual increase, from 5.2% in 2002-2003 to 30.6% in 2010-2011 (p<0.001). Proportions of patients who met all three clinical predictive parameters-female, adenocarcinoma, and never-smoker-increased by year from 13.8% to 47.7% (p<0.001).
Since EGFR mutation testing was first performed, clinical needs for EGFR mutation testing have increased, and the number of EGFR mutation tests performed has shown a rapid increase, from 2 to 197. In the year 2010-2011, 73.5% of patients who received gefitinib underwent EGFR mutation testing, and gefitinib was prescribed based on the results of the EGFR mutation test.
3. Clinical outcomes of gefitinib by year
In the year 2002-2003, the response rate was only 17.2%. However, response rate showed a steady increase, from 17.2% in 2002-2003, to 18.7% in 2004-2005, 30.2% in 2006-2007, 45.2% in 2008-2009, and 57.1% in 2010-2011 (p<0.001). The median PFS of gefitinib also increased with statistical significance from 2.8 months in 2002-2003 to 9.1 months in 2010-2011 (p<0.001). With prolongation of PFS, OS has also increased by year (p<0.001). In Fig. 1, Kaplan-Meier survival curves show a prolonged PFS of gefitinib and OS by year. However, in analysis according to EGFR mutation results, the response rate and PFS did not differ by year in both EGFR mutation positive and negative subgroups (Table 3). The median PFS of gefitinib was 10.9 months in EGFR mutation positive patients, and 1.9 months in EGFR mutation negative patients (p<0.001). The median OS also differed by EGFR mutation status (27.9 months in mutation positive vs. 14.0 months in mutation negative; p<0.001). In calculation of OS from the time of diagnosis to death, similar results were observed and OS has also increased by year (26.6 months in 2002-2003, 20.2 months in 2004-2005, 21.3 months in 2006-2007, 26.5 months in 2008-2009, and not reached in 2010-2011; p<0.001).
Discussion
In the present study, we found that prescribing patterns of gefitinib have changed over time, and this change has resulted in real improvement in the response rate of gefitinib and survival prolongation in NSCLC. Discovery of EGFR mutation in 2004 and results of several clinical trials [14-21] supporting the correlation between gefitinib and EGFR mutation have allowed clinicians to optimize and select patients who are likely to respond to gefitinib. Gefitinib tends to be prescribed upfront for first line use, based on the clinical predictor and EGFR mutation test.
Development of gefitinib and discovery of EGFR mutation were innovative turning points that proclaimed the beginning of molecular targeted agents and personalized chemotherapy, based on molecular biomarkers. Fig. 2 shows pivotal landmark events in the history of gefitinib. In the era of the targeted agent, understanding of molecular targets and tumor biomarkers is essential. Although findings of clinical trials have suggested that remarkable advances have been made with development of gefitinib in treatment of NSCLC, it is not clear whether clinical outcome of these patients has improved in the context of daily practice.
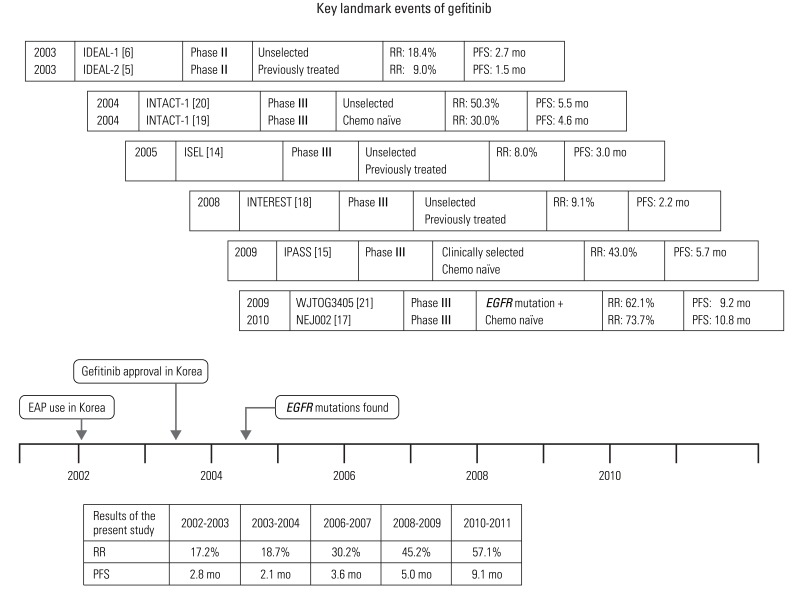
Key landmark events of gefitinib. EGFR, epidermal growth factor receptor; RR, response rate; PFS, progression-free survival; EAP, expanded access program.
According to our results, response rate and PFS showed a gradual increase, from 17.2% and 2.8 months, in 2002-2003 to 57.1% and 9.1 months in 2010-2011, respectively (p<0.001 and p<0.001). Not only the response rate and PFS, but also OS showed significant improvement by year (p<0.001). Survival improvement from treatment with gefitinib was consistent with that of the previous report [23]. Takano et al. [23] compared a gefitinib treated EGFR mutation positive patients with a historic control group comprised of EGFR mutation positive patients without gefitinib treatment. Gefitinib yielded a survival benefit in Japanese lung cancer patients and significantly greater OS benefit was observed in patients with EGFR mutations than in those without mutation [23].
Over time, clinicians tend to prescribe upfront first line use and with precise optimization based on the clinical selection or the EGFR mutation test. These trends demonstrate that biomarkers can be rapidly adapted from bench to clinic, and further therapeutic benefits can be optimized. In our results, gefitinib was prescribed non-selectively before 2004. Then, with discovery of EGFR mutation and clinical parameters, gefitinib was prescribed based on the clinical selection, and, eventually, prescription of gefitinib has tended to be based on the results of EGFR mutation test. Improvement of survival appears to result from judicious selection of patients based on molecular understanding. In our results, response rate and PFS were not improved by year in the EGFR mutation positive subgroup, as well as the EGFR mutation negative subgroup.
The current study has some potential limitations. First, our study was conducted in only a single institution. The feasibility and capacity for EGFR mutation testing and institutional policy for mutation screening strategy can vary by institution. Second, EGFR mutation positivity rate, smoking, and genetic profiles have been shown to differ between Asian and Western countries [24]. Our results could not be generalized to Western countries. Third, other factors could mediate improvements in OS over time. These include improvements in supportive care and subsequent chemotherapy after gefitinib. Beside gefitinib, introduction of other novel agents, such as pemetrexed can also contribute to improvement of OS, especially in nonsquamous cell carcinoma. Not only appropriate use of gefitinib according to EGFR mutation status, but also improvement of treatment for brain metastasis, including stereotactic radiosurgery can lead to improved OS. Fourth, we used the Sanger sequencing method [22] for detection of EGFR mutation, which showed low sensitivity in comparison with other methods [25]. There might be possibility of false negative result for EGFR mutation. Despite these limitations, to the best of our knowledge, this study was the first study to demonstrate that molecular understanding can lead to a direct change in the prescribing patterns and clinical outcome of gefitinib in outside clinical trials.
Conclusion
We found that molecular understanding and practical use of EGFR mutation testing have changed the prescribing patterns of gefitinib, and use of an enrichment strategy can lead to improvement in the efficacy of gefitinib in real practice. Optimization for patients and use of an enrichment strategy based on biomarkers translate directly into real improvement of treatment efficacy in the era of molecular targeted agents. This finding emphasizes the importance of molecular understanding and use of validated biomarkers for molecular targeted agents.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (2010-0009563). We would especially like to thank our database manager, Ju Youn Kim, for her data management. We also wish to thank the members of the Seoul National University Hospital Lung Study Group.
Notes
Conflict of interest relevant to this article was not reported.



