Intrathoracic Progression Is Still the Most Dominant Failure Pattern after First-Line Chemo-immunotherapy in Extensive-Stage Small-Cell Lung Cancer: Implications for Thoracic Radiotherapy
Article information
Abstract
Purpose
This study aimed to compare the failure patterns before and after the introduction of immunotherapy and to determine the role of thoracic radiotherapy (TRT) in extensive-stage small-cell lung cancer (ES-SCLC) treatment.
Materials and Methods
We retrospectively reviewed 294 patients with ES-SCLC, of which 62.2% underwent chemotherapy alone, 13.3% underwent chemotherapy followed by consolidative TRT (TRT group), and 24.5% underwent chemotherapy with immune checkpoint inhibitor (ICI group). We performed propensity-score matching (PSM) to compare each treatment group.
Results
The median follow-up duration was 10.4 months. At the first relapse, in the cohort showing objective response, the proportion of cases showing intrathoracic progression was significantly lower in the TRT group (37.8%) than in the chemotherapy-alone (77.2%, p < 0.001) and the ICI (60.3%, p=0.03) groups. Furthermore, in the subgroup analysis, TRT showed benefits related to intrathoracic progression-free survival (PFS) in comparison with ICI in patients with less than two involved extrathoracic sites (p=0.008) or without liver metastasis (p=0.02) or pleural metastasis (p=0.005) at diagnosis. After PSM, the TRT group showed significantly better intrathoracic PFS than both chemotherapy-alone and ICI groups (p < 0.001 and p=0.04, respectively), but showed no significant benefit in terms of PFS and overall survival in comparison with the ICI group (p=0.17 and p=0.31, respectively).
Conclusion
In ES-SCLC, intrathoracic progression was the most dominant failure pattern after immunotherapy. In the era of chemoimmunotherapy, consolidative TRT can still be considered a useful treatment strategy for locoregional control.
Introduction
Small-cell lung cancer (SCLC) accounts for 14% of all lung cancers and exhibits the most aggressive behavior, which is associated with rapid tumor growth and early distant metastasis [1]. Two-thirds of patients with SCLC present with extensive-stage SCLC (ES-SCLC) at the time of diagnosis [2,3]. For decades, the cornerstone of treatment in patients with ES-SCLC has been 4-6 cycles of platinum-based chemotherapy and etoposide [4]. Although combination chemotherapy can improve short-term survival, long-term survival remains poor [5]. Most patients eventually experience disease progression, and the 2-year survival rate is less than 5% [1].
Several phase 3 randomized controlled trials and meta-analyses have evaluated the role of consolidative thoracic radiotherapy (TRT) in patients with ES-SCLC who responded to first-line chemotherapy [6-11]. These studies revealed that chemotherapy followed by TRT significantly improved local control, progression-free survival (PFS), and overall survival (OS) in selected patients [6-11]. As a result, in the chemotherapy era, consolidative TRT became a standard of care in patients with ES-SCLC who showed a favorable response to chemotherapy.
More recently, several phase 3 studies have demonstrated significant survival benefits with a combination of chemotherapy and immune checkpoint inhibitor (ICI) therapy in ES-SCLC [12-14]. Therefore, current guidelines recommend chemoimmunotherapy as the first-line treatment for ES-SCLC [15,16]. However, the role of TRT in the era of chemoimmunotherapy has not yet been evaluated because the studies that led to the introduction of ICI did not permit the use of consolidative TRT [12-14].
Therefore, this study aimed to investigate the changes in the patterns of failure before and after the introduction of immunotherapy in ES-SCLC and to determine the role of TRT in the era of chemoimmunotherapy.
Materials and Methods
1. Study population
We retrospectively reviewed the medical records of 396 patients who were diagnosed as showing ES-SCLC at two institutions from January 2016 to June 2021. At the initial diagnosis, F-18 fluorodeoxyglucose positron emission tomography (FDG-PET)/computed tomography (CT) and brain magnetic resonance imaging (MRI) were performed to evaluate the patients. The exclusion criteria for this study were as follows: less than 3 months of follow-up; treatment with only the best supportive care; and history of other malignancies within 5 years, excluding in situ tumors of the breast or uterine cervix, differentiated thyroid cancer, and basal cell carcinoma. In addition, five patients who underwent consolidative TRT after chemoimmunotherapy were excluded. Ultimately, 294 patients were included in the present study (S1 Fig.).
2. Treatment
In total, 62.2% (n=183) of the patients underwent chemotherapy alone, 13.3% (n=39) underwent chemotherapy followed by consolidative TRT (TRT group), and 24.5% (n=72) underwent chemotherapy with ICI without TRT (ICI group). The chemotherapy regimen was a combination of platinum agents (carboplatin or cisplatin) and etoposide in all patients. Carboplatin and cisplatin were administered to 63.6% and 36.4% of the patients, respectively. The median number of chemotherapy cycles in both the chemotherapy-alone and TRT groups was 6 (interquartile range [IQR], 4 to 6). The decision to administer consolidative TRT was made collaboratively between the hemato-oncology and radiation oncology departments. Consolidative TRT was mainly performed in patients who showed an objective response (complete response [CR] or partial response [PR]) after chemotherapy. The planning target volume was based on the post-chemotherapy volume with a 10- to 15-mm margin considering respiratory movements, microscopic disease, and setup errors. In cases showing CR after chemotherapy, the initially involved lymph nodes were included in the target volume. Furthermore, any residual lesions from multiple metastatic lung nodules post-chemotherapy were also included in the TRT field. However, the initially involved extrathoracic lesions were not included in the irradiated field. TRT was delivered as a median dose of 50 Gy (IQR, 45 to 54 Gy) over a median of 25 fractions (IQR, 15 to 27 fractions) with three-dimensional conformal radiotherapy (RT) (n=7, 17.9%) or intensity-modulated RT (n=32, 82.1%). TRT was administered with 6-10 MV photons from linear accelerators and all fields were treated daily (five fractions per week). TRT was performed for a median of 46 days (IQR, 34 to 53 days) after chemotherapy. Prophylactic cranial irradiation (PCI) was administered to 24 patients at doses of either 25 Gy in 10 fractions or 20 Gy in 5 fractions. PCI was given based on physician judgment, specifically for patients without brain metastases on the initial brain MRI who also maintained a good performance status following their scheduled initial chemotherapy. At our institution, we favored active brain MRI surveillance over PCI; therefore, most patients did not receive PCI. Palliative TRT was conducted among patients showing intrathoracic disease progression after first-line therapy according to the judgment of a radiation oncologist, and it was delivered at a median dose of 39 Gy (IQR, 20 to 50 Gy) over a median of 15 fractions (IQR, 5 to 20 fractions). ICI for ES-SCLC was introduced to our institution in 2020, and anti–programmed death-ligand 1 inhibitors (atezolizumab and durvalumab) were administered for ICI. Atezolizumab and durvalumab were administered in 94.4% (n=68) and 5.6% (n=4) of the patients, respectively. In the induction phase, the patients in the ICI group concurrently received platinum-based doublet chemotherapy with ICI; the median number of induction cycles was 4 (IQR, 2 to 6). The induction phase was followed by a maintenance phase in which patients continued to receive ICI until evidence of disease progression or intolerable toxicity was observed; the median number of maintenance cycles was 4 (IQR, 2 to 7). In the ICI group, consolidative TRT was administered during or before the maintenance phase, and only five patients were treated; however, as mentioned above, these patients were excluded from the survival analysis due to the small sample size.
3. Follow-up and assessment
Response to the initial treatment was evaluated by clinical examination and chest/abdominal CT every 2-3cycles of the initial chemotherapy with or without ICI. Subsequently, routine follow-up assessments were conducted every 3 months. At every visit, the patients underwent blood and biochemical tests, chest radiography, CT, and brain MRI. FDG-PET/CT and fine-needle aspiration biopsy were performed as required. Tumor response to initial treatment was assessed by the physicians with the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 [17].
4. Statistical analysis
The chi-square test or Fisher’s exact test were performed to compare variables according to treatment group. We performed propensity-score matching (PSM) analysis to control for differences in characteristics between treatment groups. The variables used in PSM were as follows: age, sex, European Cooperative Oncology Group performance status, smoking status, duration of follow-up, response to systemic therapy, number of involved extrathoracic sites, and metastasis status in the brain, bone, liver, and pleura at diagnosis. The matched cohorts were constructed using 2:1 (chemotherapy-alone group/TRT group), 2:1 (chemotherapy-alone group/ICI group), and 1:1 (TRT group/ICI group) ratios for each comparison group, respectively. Using propensity scores, each treatment group was matched with a nearest-neighbor matching algorithm using a caliper width of 0.5 standard deviations. Intrathoracic progression was defined as tumor progression or newly developed lesions in the lung, mediastinal lymph nodes, or pleura. The primary endpoints were intrathoracic PFS and PFS. Intrathoracic PFS was defined as the time from diagnosis to intrathoracic disease progression, death, or the last follow-up. PFS was defined as the time from diagnosis to any disease progression, death, or the last follow-up. The secondary endpoint was OS, which was calculated from the date of diagnosis to all-cause mortality. Survival outcomes were calculated by Kaplan-Meier analysis and comparisons between treatment groups were performed by log-rank test. We assessed grade 3 or higher esophageal and bronchopulmonary toxicities related to TRT using the Common Terminology Criteria for Adverse Events (ver. 5.0). We defined statistical significance as a p < 0.05. All statistics were evaluated using STATA software ver. 17.0 (Stata Corp., College Station, TX).
Results
1. Patient characteristics
Table 1 presents the patient characteristics of the entire cohort according to treatment groups. The median patient age was 68 years (IQR, 48 to 84 years). The distribution of the number of involved extrathoracic sites, liver metastasis at diagnosis, follow-up duration, and response to systemic therapy differed across treatment groups. The number of patients with fewer than two extrathoracic sites involved, no liver metastasis at diagnosis, longer follow-up duration, and objective response (PR or CR) to systemic therapy was significantly greater in the TRT group than in the other treatment groups. However, the treatment groups showed no significant differences for the other variables.
After initial chemotherapy, the proportion of patients who experienced a worsening in Eastern Cooperative Oncology Group (ECOG) performance status was 26.2% (n=48) in the chemotherapy-alone group, 15.4% (n=6) in the TRT group, and 25.0% (n=18) in the ICI group. Notably, the TRT group exhibited a lower proportion of patients with deteriorating performance status post-initial systemic therapy than the other groups. Regarding the completion rate of scheduled initial chemotherapy, it was 79.8% for the chemotherapy-alone group, 92.3% for the TRT group, and 87.5% for the ICI group. As for dose reductions during chemotherapy, the rate was 34.4% (with reductions of 20%-40%) for the chemotherapy-alone group, 12.8% (with reductions of 20%-30%) for the TRT group, and 31.9% (with reductions of 20%-30%) for the ICI group. Fewer patients in the TRT group underwent a dose reduction compared to the other groups.
2. Patterns of progression and treatment outcomes
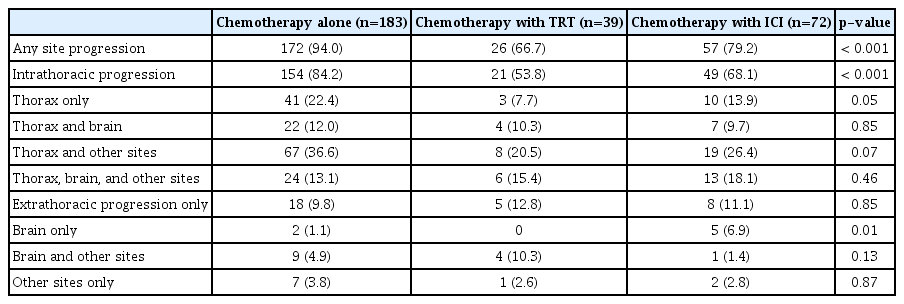
The median follow-up duration was 10.4 months (IQR, 6.9 to 16.1 months). The patterns of progression in the entire cohort are described in Table 2. During the follow-up period, disease progression occurred in 208 patients (84.9%): 172 in the chemotherapy-alone group, 57 in the ICI group, and 26 in the TRT group. The chemotherapy-alone group (94.0%) had a significantly higher proportion of patients experiencing any disease progression than the TRT group (66.7%, p < 0.001) and ICI group (79.2%, p < 0.001). Furthermore, the chemotherapy-alone group (84.2%) had a significantly higher proportion of patients showing intrathoracic disease progression than the TRT group (53.8%, p < 0.001) and ICI group (68.1%, p=0.004); however, the TRT and ICI groups did not show a significant difference (p=0.14). The proportion of patients showing intrathoracic disease progression among all disease events was 89.5% (154/172) in the chemotherapy-alone group, 80.8% (21/26) in the TRT group, and 86.0% (49/57) in the ICI group. However, no significant difference was observed across the three groups (p=0.39). Further details on the patterns of intrathoracic progression are provided in S2 Table. Of the total intrathoracic progressions (n=224), 92.4% (n=207) occurred from the initial thoracic lesion. When categorized by group, the TRT group exhibited a lower rate of disease progression from the initial intrathoracic lesion, at 66.7% (14/21), compared to the chemotherapy-alone group at 96.7% (149/154) and the ICI group at 89.8% (44/49).
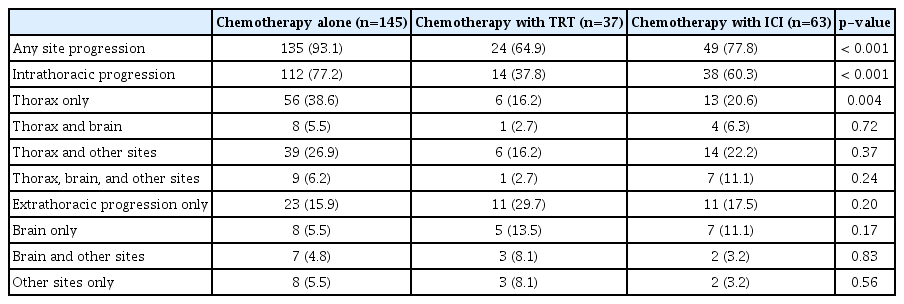
After first-line systemic therapy, 245 patients achieved an objective response according to the RECIST criteria. The patterns of the first site of failure in these patients are shown in Table 3. At the first progression, the proportion of cases showing intrathoracic disease progression was 77.2% in the chemotherapy-alone group, 37.8% in the TRT group, and 60.3% in the ICI group; it was significantly lower in the TRT group than in the chemotherapy-alone group and the ICI group (p < 0.001 and p=0.03, respectively). Furthermore, the proportion of cases showing intrathoracic progression among all events at the first relapse was 83.0% (112/135) in the chemotherapy-alone group, 58.3% (14/24) in the TRT group, and 77.6% (38/49) in the ICI group; the proportion in the TRT group was significantly lower than that in the chemotherapy-alone group (p=0.006) and showed a marginal significance in comparison with that in the ICI group (p=0.08).
The median intrathoracic PFS and PFS were 5.8 months and 5.4 months in the chemotherapy-alone group, 11.0 months and 9.2 months in the TRT group, and 6.9 months and 6.0 months in the ICI group, respectively. The 6-month and 1-year intrathoracic PFS were 51.4% and 17.0% in the chemotherapy-alone group, 94.9% and 64.3% in the TRT group, and 60.3% and 34.9% in the ICI group, respectively. The 6-month and 1-year PFS were 39.3% and 6.0% in the chemotherapy-alone group, 87.2% and 30.8% in the TRT group, and 50.0% and 24.9% in the ICI group, respectively. The Kaplan-Meier curves for intrathoracic PFS and PFS showed significant differences across the three groups (all p < 0.005) (Fig. 1A and B). The median OS of the chemotherapy-alone, TRT, and ICI groups was 9.8, 16.4, and 11.6 months, respectively. The 6-month and 1-year OS were 79.8% and 37.2% in the chemotherapy-alone group, 97.4% and 66.7% in the TRT group, and 83.3% and 54.6% in the ICI group, respectively. The Kaplan-Meier curves for OS showed significant differences in both TRT and ICI groups in comparison with the chemotherapy-alone group (p < 0.001 and p=0.01, respectively) (S3 Fig.); the TRT group showed a trend of better OS than the ICI group (p=0.08) (S3 Fig.).
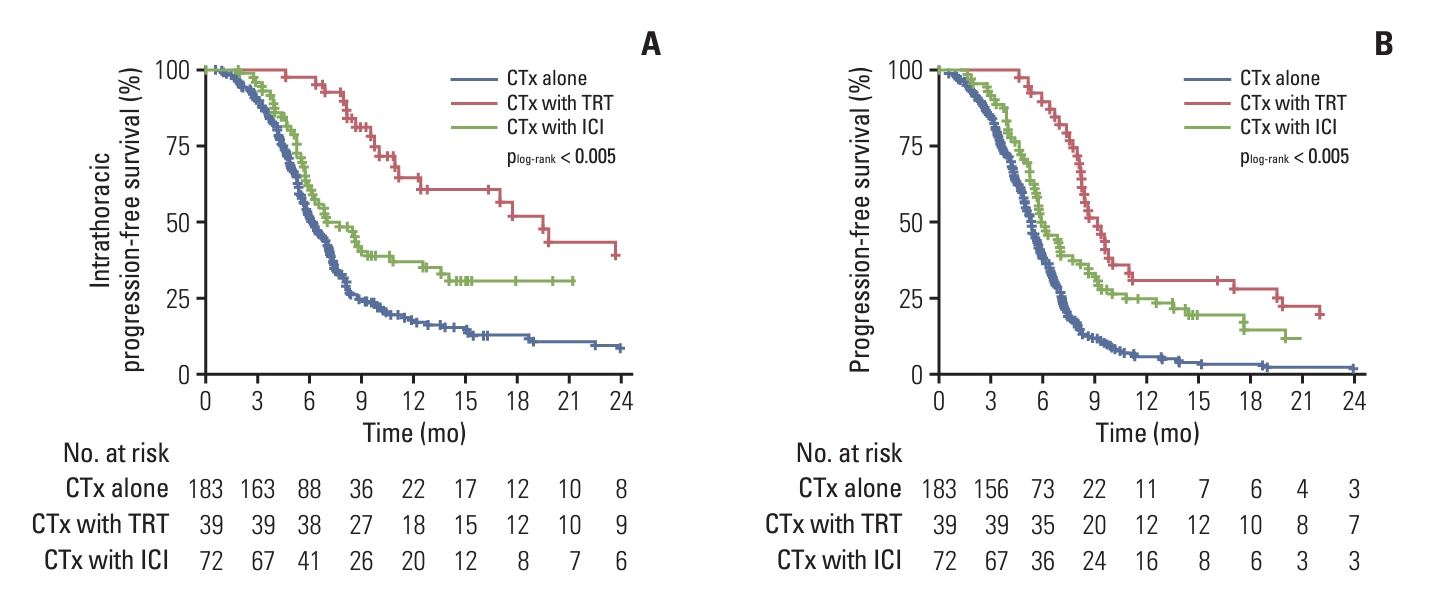
Kaplan-Meier curves of intrathoracic progression-free survival (A) and progression-free survival (B) according to the treatment group in the entire cohort. CTx, chemotherapy; ICI, immune checkpoint inhibitor; TRT, thoracic radiotherapy.
Palliative TRT was not performed in patients who received consolidative TRT but was performed in 7.7% and 15.3% of patients in the chemotherapy-alone group and ICI group, respectively (S4A Fig.). In the cohort with an objective response, palliative TRT was performed in 5.5% and 12.7% of the patients in the chemotherapy-alone and ICI groups, respectively (S4A Fig.). In both the entire cohort and the objective response cohort, the patients in the ICI group tended to receive more palliative TRT than those in the TRT group, but the difference was not statistically significant (entire cohort: p=0.06; objective response cohort: p=0.07). In addition, consolidative TRT was performed less in the chemoimmu-notherapy era than in the chemotherapy era. Indeed, in the entire cohort, the proportion of patients receiving consoli-dative TRT decreased from 17.6% to 6.5% after the introduction of immunotherapy (S4B Fig.). Moreover, among patients who achieved an objective response, consolidative TRT was performed in 20.3% of the patients in the chemotherapy era and 7.4% of those in the chemoimmunotherapy era (S4B Fig.).
Treatment for initial brain metastasis or brain disease progression was performed in a total of 57 patients. Of these, 27 were in the chemotherapy-alone group, 15 in the TRT group, and 15 in the ICI group. The treatment modalities for these patients included: whole brain radiotherapy (n=41), whole brain radiotherapy after craniotomy (n=2), whole brain radiotherapy after Gamma knife radiosurgery (n=5), craniotomy (n=1), and Gamma knife radiosurgery (n=8).
Regarding treatment–related toxicities, in the TRT group, grade 3 esophagitis and pneumonitis were observed in one (2.6%) and two (5.1%) patients, respectively; no patient experienced toxicities exceeding grade 3. In both the chemotherapy-alone group and the ICI group, there were no cases of esophagitis of grade 3 or higher. Grade 3 pneumonitis was observed in three patients (1.6%) from the chemotherapy-alone group and two patients (2.8%) from the ICI group; among the patients with pneumonitis in the chemotherapy-alone group, one had received palliative TRT.
3. Subgroup analyses
For comparisons between the chemotherapy-alone and TRT groups, 76 and 38 patients were included after 2:1 PSM, respectively. The patient characteristics of the matched cohort are summarized in S5 Table. In the matched cohort, the proportion of cases showing intrathoracic progression in the TRT group was significantly lower than that in the chemotherapy-alone group (39.5% vs. 76.3%, p < 0.001). The median intrathoracic PFS and PFS were 5.8 months and 5.3 months in the chemotherapy-alone group and 9.9 months and 8.4 months in the TRT group of the matched cohort, respectively. The TRT group showed significantly better intrathoracic PFS and PFS than the chemotherapy-alone group (all p < 0.001) (Fig. 2A and B).
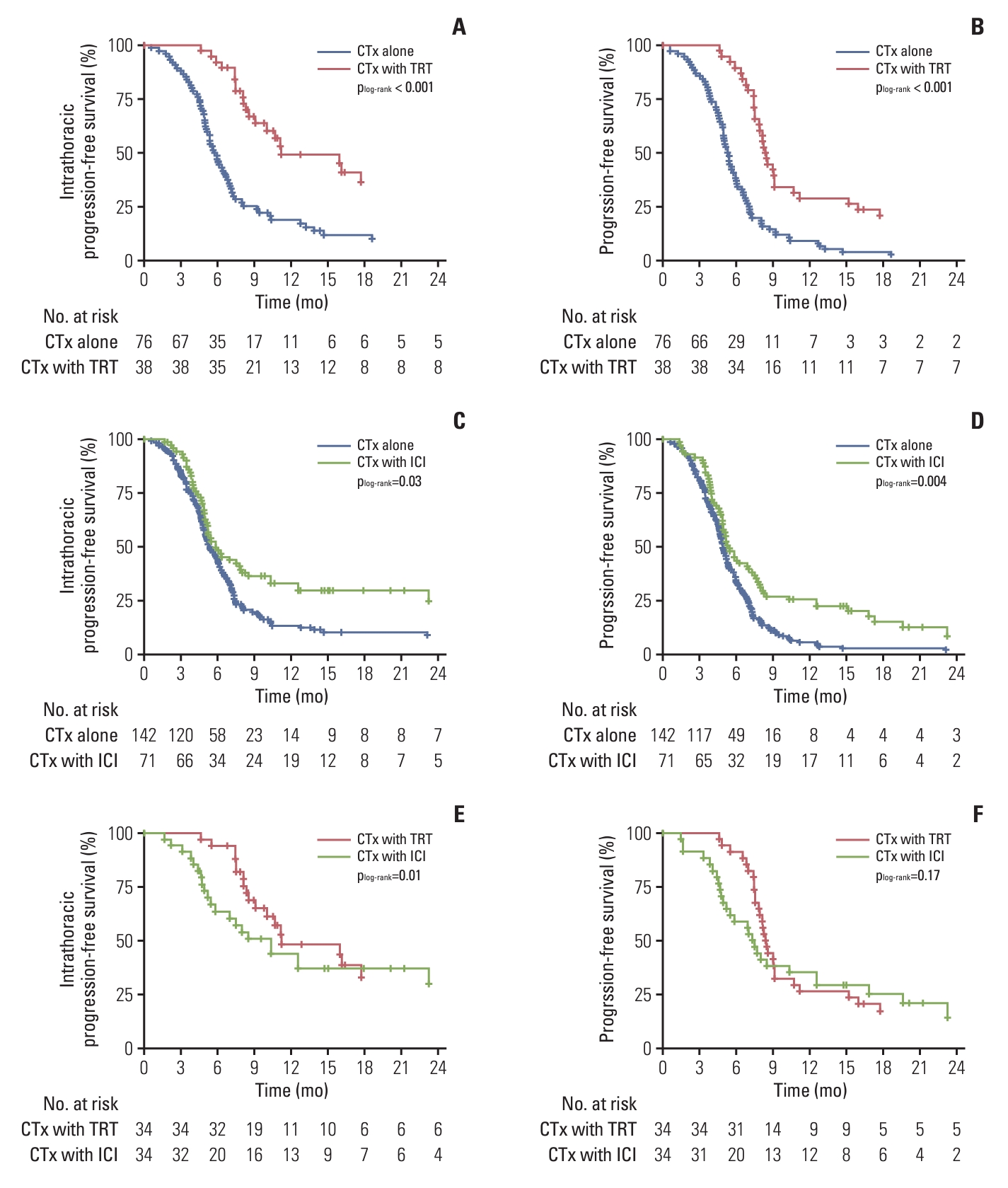
Kaplan-Meier curves of intrathoracic progression-free survival and progression-free survival in the propensity-score matched cohorts. The chemotherapy-alone and the TRT groups were matched in a 2:1 ratio, and intrathoracic progression-free survival (A) and progression-free survival (B) were compared. The chemotherapy-alone and the ICI groups were matched in a 2:1 ratio, and intrathoracic progression-free survival (C) and progression-free survival (D) were compared. The TRT and the ICI groups were matched in a 1:1 ratio, and intrathoracic progression-free survival (E) and progression-free survival (F) were compared. CTx, chemotherapy; ICI, immune checkpoint inhibitor; TRT, thoracic radiotherapy.
For comparison between the chemotherapy-alone and the ICI groups, 118 and 59 patients were included after 2:1 PSM, respectively. The patient characteristics of the matched cohort are summarized in S6 Table. In the matched cohort, the proportion of cases showing intrathoracic progression in the ICI group tended to be lower than that in the chemotherapy-alone group (62.7% vs. 75.4%, p=0.08). The median intrathoracic PFS and PFS were 5.5 months and 5.3 months in the chemotherapy-alone group and 6.0 months and 5.8 months in the ICI group of the matched cohort, respectively. The ICI group showed significantly better intrathoracic PFS and PFS than the chemotherapy-alone group (p=0.03 and p=0.004, respectively) (Fig. 2C and D).
For comparisons between the TRT and the ICI groups, 32 patients were included in each group after 1:1 PSM. The patient characteristics of the matched cohort are summarized in S7 Table. In the matched cohort, the proportion of patients showing intrathoracic progression in the TRT group was significantly lower than that in the ICI group (34.4% vs. 59.4%, p=0.04). The median intrathoracic PFS and PFS in the matched cohort were 10.3 months and 8.3 months in the TRT group and 7.3 months and 7.0 months in the ICI group, respectively. The TRT group showed a significantly better intrathoracic PFS than the ICI group (p=0.01) (Fig. 2E); however, PFS showed no significant difference between the two groups (p=0.17) (Fig. 2F).
After PSM, OS was significantly better in both TRT and ICI groups than in the chemotherapy-alone group (p=0.001 and p=0.02, respectively) (S8A and S8B Fig.), but the difference in OS between the TRT and ICI groups was not statistically significant (p=0.31) (S8C Fig.). Additionally, we roughly compared OS between the patients in the TRT and ICI groups who received palliative TRT; the patients in the TRT group showed marginally better OS than those in the ICI group who received palliative TRT (p=0.06) (S9 Fig.).
We also performed subgroup analyses of patients who achieved an objective response in the TRT and ICI groups to identify a subset of patients who might benefit more from TRT (Fig. 3). In terms of intrathoracic PFS, TRT showed benefits in patients with less than two involved extrathoracic sites (p=0.008) or without liver metastasis (p=0.02) or pleural metastasis (p=0.005) at diagnosis (Fig. 3A); meanwhile, TRT showed superior outcomes compared to ICI even in patients with brain metastasis (Fig. 3A). In terms of PFS, TRT showed better outcomes in patients without pleural metastasis (Fig. 3B).
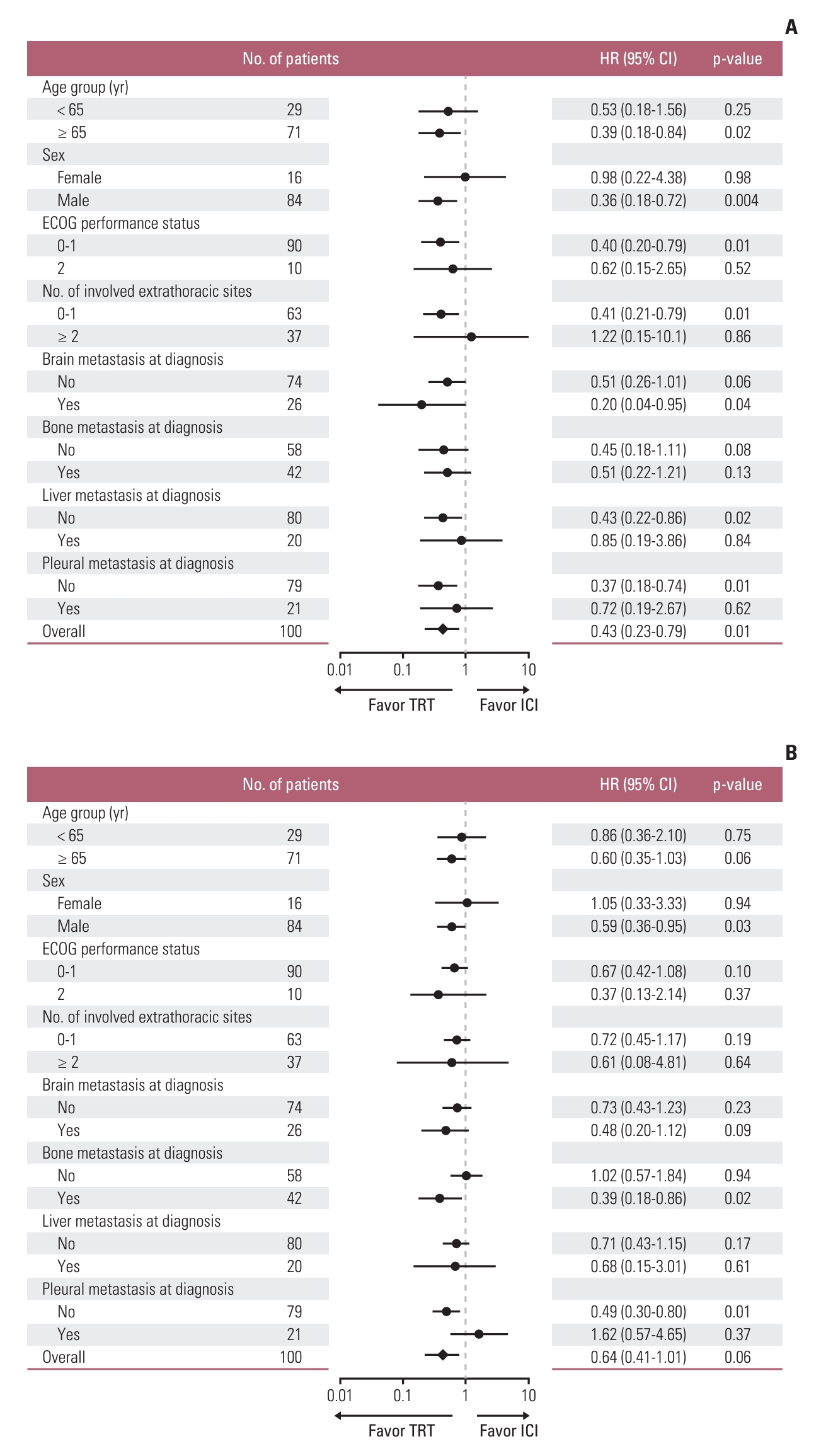
Intrathoracic progression-free survival (A) and progression-free survival (B) according to subgroup analyses between the TRT and ICI groups in the cohort with an objective response. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; ICI, immune checkpoint inhibitor; TRT, thoracic radiotherapy.
Discussion
In patients with ES-SCLC, chemotherapy significantly prolonged the survival rate, but the median survival times were limited to 9-12 months [18,19]. In particular, the main problem in ES-SCLC patients undergoing chemotherapy was intrathoracic tumor progression, and approximately 90% of patients have been reported to develop intrathoracic progressive disease within 1 year after diagnosis [19]. Several recent studies have demonstrated that a combination of chemotherapy with immunotherapy improved disease control and survival outcomes better than chemotherapy alone [12-14]. In these studies, 80%-85% of patients showed lung or mediastinal lymph node involvement at diagnosis, and only 2.5% achieved CR after chemoimmunotherapy [12].
Indeed, in our study, chemoimmunotherapy yielded significantly better treatment outcomes than chemotherapy alone, consistent with the results of other studies on ES-SCLC. Nevertheless, our results revealed that intrathoracic progression was the most dominant pattern of failure for the first relapse and overall tumor progression even after chemoimmunotherapy; besides, these failure patterns did not show significant differences in comparison with chemotherapy alone. Meanwhile, patients in the ICI group tended to receive more palliative TRT than those in the chemotherapy-alone group. This can be explained by the fact that consolidative TRT was performed more frequently in the chemotherapy era. Additionally, in a rough comparison of our results, the TRT group tended to have better OS than patients in the ICI group who received palliative TRT. These results might suggest that early intervention of consolidative TRT, rather than palliative TRT after intrathoracic progression, probably gives the survival benefit in patients treated with chemoimmunotherapy, although we could not show treatment outcomes of chemoimmunotherapy with consolidative TRT. Therefore, given the high rate of intrathoracic failure even after chemoimmunotherapy, management techniques for locoregional control, such as consolidative TRT, in selected patients should still be considered.
In the era of chemotherapy, several prospective studies and meta-analyses have demonstrated that consolidative TRT offers survival benefits in comparison with chemotherapy alone [6-11]. Slotman et al. [7], in a phase 3 randomized controlled study, reported that the 2-year OS significantly improved from 3% to 13% when consolidative TRT was performed in ES-SCLC patients who responded to chemotherapy; the intrathoracic progression was significantly lower in the chemotherapy with TRT than the chemotherapy-alone group (44% vs. 80%). Additionally, a follow-up study found significantly better survival outcomes when TRT was performed in patients with two or fewer distant metastases [20]. Accordingly, the authors suggested that more intensive TRT with a curative aim should be considered in ES-SCLC patients with a low tumor burden [20].
In our study, consistent with previous studies, TRT significantly reduced intrathoracic tumor progression in comparison with chemotherapy alone; this beneficial effect of TRT was more prominent in patients who achieved an objective response. Notably, in our study, the TRT group exhibited significantly better intrathoracic tumor control and intrathoracic PFS than the ICI group, even after PSM. In addition, among patients with objective response, TRT showed better intrathoracic PFS than ICI in subsets with a low initial tumor burden (without liver or pleural metastasis and less than two extrathoracic sites). We also observed that intrathoracic progression was predominantly observed in the initial thoracic lesion for both the chemotherapy-alone and ICI groups. While the TRT group exhibited better control of initial intrathoracic lesions than the other two groups, even though only the post-chemotherapy volume was set as the TRT field. Moreover, the incidence of TRT-related toxicity was consistent with findings from previous other studies and was deemed tolerable [7]. These findings suggest that TRT plays a complementary role in addition to chemoimmunotherapy in ES-SCLC.
To date, data from phase 3 randomized controlled trials evaluating the effect of TRT in the era of chemoimmunotherapy are limited; therefore, many institutions, including ours, are hesitant to use the combination of chemoimmunotherapy with TRT as first-line treatment for ES-SCLC. On the other hand, immunotherapy has been successfully used with TRT for advanced non-SCLC [21,22]. Recent retrospective studies have evaluated the safety and efficacy of the combination of immunotherapy with TRT in ES-SCLC [23,24]. Galuba et al. [23] reported that the addition of TRT to immunotherapy did not appear to be associated with increased toxicity in comparison with TRT alone in patients with SCLC. Diamond et al. [24] suggested that chemoimmunotherapy followed by consolidative TRT is safe and feasible with a median PFS and OS of 6.7 months and 16 months, respectively, which were comparable to the findings reported in published modern clinical trials. Although these retrospective studies were small and did not evaluate long-term outcomes, the results showing the safety of the combination of TRT and immunotherapy should encourage further studies. Additionally, several preclinical and clinical studies have reported that the combination of radiotherapy and immunotherapy can enhance local control and show systemic anti-tumor immunity as a synergistic effect [25,26]. These results lead us to expect promising results from ongoing prospective trials evaluating the combination of chemoimmunotherapy with TRT for ES-SCLC.
The RAPTOR/NRG LU007 trial is a randomized phase 2/3 study that will evaluate the PFS and OS of ES-SCLC patients receiving TRT after first-line chemoimmunotherapy with atezolizumab [27]. The TREASURE trial is a randomized phase 2 trial that will investigate whether the addition of TRT to atezolizumab maintenance therapy can improve OS in patients showing a response after chemoimmunotherapy [28]. These ongoing trials will answer important clinical questions regarding the need for TRT in the chemoimmunotherapy era. However, randomized evidence from these studies, which have recently started enrollment, will only arrive after some time. Moreover, as mentioned above, coincident with the reduction in the use of consolidative TRT, the proportion of patients requiring palliative TRT tended to increase in the chemoimmunotherapy era. Therefore, our study tried to guide the clinical practices and elucidate the role of TRT until results from these trials became available.
The main strength of this study is that, to the best of our knowledge, it is the first study to compare the failure patterns of chemotherapy-alone, consolidative TRT, and chemoimmunotherapy and also provides potential insights into the role of TRT after chemoimmunotherapy in patients with ES-SCLC. However, our study has some limitations worth consideration. First, this study had a retrospective design, and selection bias could not be completely avoided. Indeed, the TRT group included more patients who responded favorably to chemotherapy and had a lower tumor burden than the other groups. Therefore, we independently conducted PSM analysis for each treatment modality and performed subgroup analysis in the cohort that achieved an objective response to minimize the imbalance between groups. However, due to incomplete control over various detailed clinical factors—such as differences in performance status post-chemotherapy, chemotherapy completion rates, and dose reduction rates—the possibility of selection bias still remains. Thus, caution should be exercised when interpreting our results. Second, we did not directly evaluate the effect of combining chemoimmunotherapy with TRT. This is because chemoimmunotherapy for ES-SCLC was recently introduced at our institution and few patients had received additional consolidative TRT. Instead, we analyzed the change in the pattern of failure and treatment outcome in cohorts matched by each treatment group, indicating the need for investigations of the role of TRT in the immunotherapy era. Lastly, the sample sizes of the TRT and ICI groups were insufficient for detailed subgroup analysis. Therefore, further large-scale studies are warranted to validate our results.
In conclusion, our results indicated that intrathoracic tumor progression was the dominant pattern of failure even after chemoimmunotherapy in patients with ES-SCLC. Consolidative TRT administered following chemotherapy resulted in significantly reduced intrathoracic tumor progression and improved intrathoracic PFS in comparison with chemotherapy alone and chemoimmunotherapy without TRT. In the era of chemoimmunotherapy, consolidative TRT can still be considered a useful treatment strategy for locoregional disease control.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the institutional review boards of both hospitals (Hospital A, IRB No. 20-2022-43; Hospital B, IRB No. 2205-132-1327). The requirement for informed consent was waived owing to the retrospective nature of the study.
Author Contributions
Conceived and designed the analysis: Kim D, Kim HJ, Wu HG, Lee JH, Kim S, Kim TM, Kim JS, Kim BH.
Collected the data: Kim D, Kim HJ, Wu HG, Lee JH, Kim S, Kim TM, Kim JS, Kim BH.
Contributed data or analysis tools: Kim D, Kim HJ, Wu HG, Lee JH, Kim S, Kim TM, Kim JS, Kim BH.
Performed the analysis: Kim D, Kim BH.
Wrote the paper: Kim D, Kim BH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.