AbstractPurposeGallbladder cancer is a malignancy with poor prognosis, predominantly resulting from invasion and metastasis. Our previous studies have demonstrated that prostaglandin E2 (PGE2), generated by cyclooxygenase 2 (Cox-2), transactivates epidermal growth factor receptor (EGFR), c-Met and β-catenin; thus, enhancing colon cancer cell growth and invasiveness in vitro. To determine whether these findings are applicable to clinical conditions, we examined the expression and cellular localization/co-localization of Cox-2, c-Met, β-catenin, EGFR and c-erbB2 in gallbladder cancer.
Materials and MethodsThirty-five specimens of invasive gallbladder cancer, 8 in situ carcinoma and 7 adenoma specimens were immunostained with specific antibodies against Cox-2, c-Met, β-catenin, EGFR and c-erbB2. The cellular distribution, localization and colocalization were examined, and the signal intensities quantified in: a) the central area of gallbladder cancer and b) cancer cells forming the invasive front.
ResultsCox-2, c-Met, β-catenin, c-erbB2 and EGFR were over-expressed in 80, 74, 71, 62 and 11% of invasive gallbladder cancers, respectively. β-catenin was expressed in 80% of non-malignant specimens, exclusively in the cell membrane, while the cancer specimens showed cytoplasmic and/or nuclear staining. Significantly higher Cox-2, c-Met and β-catenin expressions were present in cancer cells of the invasive front than in the tumor central areas (p<0.001), and these expressions were significantly (p=0.01) associated with the invasion depth. Co-expressions of Cox-2, c-Met, β-catenin and c-erbB2 were present in 42% of the specimens in cancer cells forming the invasive front.
INTRODUCTIONGallbladder cancer is an aggressive and lethal disease. The 5-year survival rate after surgery has been reported to be between 5 and 13% (1,2). This is mainly due to the fact the majority of gallbladder carcinomas are in an advanced stage at the time of diagnosis, and metastases to the liver and regional lymph nodes are common (2). To improve the prognosis of patients with gallbladder carcinomas, more effective techniques for their early diagnosis, and a better understanding of the mechanisms involved in the invasion and metastasis, are essential.
Accumulating evidence suggests the overexpression of Cox-2, a key inducible enzyme involved in the production of prostaglandins and other eicosanoids, may play a significant role in carcinogenesis (3). The expression of Cox-2 is elevated in a variety of human malignancies and premalignant lesions (3,4), including gallbladder cancer (5). Cox-2 expression correlates closely with the depth of invasion in some cancers, such as gastric and urinary bladder (6,7). Inhibition of tumor cell growth, invasiveness and angiogenesis due to Cox-2 inhibitors further suggests an involvement of Cox-2 in tumor progression (8).
Protein tyrosine kinases play a crucial role in a variety of cell regulatory processes, such as proliferation, migration, adhesion and potential cellular transformation. Members of the type-1 ErbB family of protein kinase receptors include: epidermal growth factor receptor (EGFR), ErbB2, ErbB3 and ErbB4 (9). The overexpression of EGFR, with increased tyrosine kinase activity, has been associated with the extent of invasion and metastasis in a variety of gastrointestinal malignancies (10), including gallbladder cancer (11), and that of ErbB2 has also been reported in gallbladder adenocarcinomas (12).
In normal cells, membrane localized β-catenin links E-cadherin and α-catenin and; thus, regulates the functions of E-cadherin. β-catenin is also involved in the Wingless/Wnt signaling cascade, as a transcriptional activator, impacting on cell proliferation, polarity and migration in relation to developmental biology (13). Nuclear translocation of β-catenin was found in dedifferentiated tumor cells at the invasive front of colorectal cancer, while in central areas of the primary tumor, it was localized in the membrane and cytoplasm of tumor cells (14). Association of nuclear β-catenin with the T cell factor (TCF)/lymphoid enhancer factor (LEF) family of transcription factors promotes the expression of several proteins that plays important role in the development and progression of colorectal carcinoma (15).
The c-Met protooncogene was discovered by the ability of oncogenic Met to mediate chemically induced transformation of a human osteogenic sarcoma cell line (16). The binding of hepatocyte growth factor (HGF) to the c-Met receptor results in receptor autophosphorylation and upregulation of the c-Met kinase activity, which in turn stimulates a number of intracellular pathways involved in cell growth, migration and survival (17). In normal cells, c-Met activation is a ligand-dependent transient event, whereas in tumor cells c-Met is often constitutively active (17). Up-regulation and amplification of the c-Met receptor activation has been reported to promote invasion and metastasis of various cancers, including gallbladder cancer (17).
Recently, we reported that PGE2, generated by the Cox-2 enzyme, transactivates the EGFR and c-Met receptors, and increases tyrosine phosphorylation and the nuclear accumulation of β-catenin; events which promote colon cancer cell invasion (18,19). Moreover, HGF triggers the activation of the Cox-2 gene in some cells, e.g. gastric epithelial cells, through phosphorylation of the c-Met/HGF receptor and activation of the extracellular signal-regulated kinase 2 (ERK2) signaling pathway (20). These data strongly implicate local interactions between Cox-2, c-met, β-catenin, EGFR and c-erbB2 proteins, indicating their positive roles in tumor invasiveness. However, the expressions, co-localizations and possible local interactions of Cox-2, c-met, β-catenin, EGFR and c-erbB2 in cancer cells forming the margin of invasive gallbladder cancer remain unknown. We examined the expressions and cellular localizations of Cox-2, c-Met, β-catenin, EGFR and c-erbB2 in 43 gallbladder cancer and 7 adenomas specimens using immunohistochemistry. The expressions of Cox-2, c-met, β-catenin, EGFR and c-erbB2 in cancer cells at the invasive front; at the cancer-stromal interface, were compared with those in cancer cells of the central area.
MATERIALS AND METHODS1) MaterialsThe study was approved by both the Human Ethics Committee of Chonbuk National University Medical School and the Human Studies Subcommittee of the VA Medical Center, Long Beach. Forty-three formalin-fixed, paraffin-embedded gallbladder carcinoma tissue specimens, with adjacent non-malignant mucosa, and 7 adenoma surgical resection specimens were obtained from the Chonbuk National University Hospital Surgical Pathology Archives. The specimens were from 21 male and 29 female patients, with a mean age of 66.6 years, ranging from 45 to 88 years. The histological type of gallbladder cancer was determined according to the World Health Organization criteria (21), and the T classification based on the criteria of classification of the American Joint Committee on Cancer (AJCC) (22).
2) ImmunohistochemistryFor immunohistochemical staining, an immunoperoxidase method, with the Streptavidin-biotinylated horseradish peroxidase complex (DAKO, Carpinteria, CA) was employed. Four µm thick sections were cut from the formalin-fixed, paraffin-embedded tissue blocks. After deparaffinization, they were subjected to a microwave antigen retrieval procedure in 0.01 M sodium citrate buffer for 10 min. They were then incubated in methanol, containing 0.3% hydrogen peroxide, at room temperature for 20 minutes, to block the endogenous peroxidase. After blocking of the endogenous biotin (DAKO, Carpinteria, CA), sections were incubated with Protein Block Serum-Free (DAKO, Carpinteria, CA) at room temperature for 10 minutes, to block nonspecific staining, and then incubated for 2 hours at room temperature with either anti-Cox-2 (Santa Cruz Biotechnology, Santa Cruz, CA), c-Met (Santa Cruz Biotechnology, Santa Cruz, CA), β-catenin (BD Transduction, San Diego, CA), EGFR (DAKO, Carpinteria, CA) or c-erbB2 (DAKO, Carpinteria, CA) antibodies. After washing, the sections were incubated with a biotin-conjugated secondary antibody, at room temperature for 30 minutes, and finally with peroxidase conjugated streptavidin at room temperature for 30 minutes. The peroxidase activity was detected using the enzyme substrate 3 amino-9-ethyl carbazole. Sections treated as described above, with the exception of being incubated with Tris-buffered saline instead of the primary antibody, served as the negative controls. Sections taken from known positive colon carcinoma cases were used as positive controls. Samples with staining of at least 10% of the tumor cells were defined as positive, and the intensity of cell staining was graded according to the following scale; 0, no staining; 1+, weak staining; 2+, moderate staining; 3+ strong staining. The expressions of Cox-2, c-Met, β-catenin, EGFR and c-erbB2 in cancer cells at the invasive front, or at the cancer-stromal interface, were compared with their respective expressions in cells at the central parts of the tumor.
3) Statistical analysisValues are expressed as mean±SE. The Mann-Whitney Rank Sum test was used to determine the differences in the protein expression levels (staining intensity) in the cells of the central area of gallbladder cancer compared to the cancer cells forming the invasive front. An association between the T classification and Cox-2, c-Met, β-catenin, EGFR and c-erbB2 expressions was tested using the chi-squared test.
RESULTSThe forty-three gallbladder cancer specimens were classified as follows: 8 carcinoma in situ, 29 invasive adenocarcinomas, 2 clear cell adenocarcinomas, 2 adenosquamous carcinomas, 1 mucinous adenocarcinoma and 1 signet ring cell carcinoma. According to the AJCC classification, 8, 13, 17, 3 and 2 specimens were TIS (carcinoma in situ), T1 (tumor has invaded the lamina propria or muscle layer), T2 (tumor has invaded the perimuscular connective tissue; no extension beyond the serosa or into the liver), T3 (tumor has perforated the serosa or has directly invaded one adjacent organ or both) T4 (tumor extends more than 2 cm into the liver, and/or into two or more adjacent organs), respectively. The overexpressions of Cox-2, c-Met, β-catenin, EGFR and c-erbB2 was detected in 28 (80%), 26 (74%), 25 (71%), 4 (11%) and 22 (62%) of the 35 invasive gallbladder cancers specimens and in 3 (37%), 5 (62%), 6 (75%), 1 (12%) and 2 (25%) of the 8 in situ carcinomas specimens, respectively. The data on the Cox-2, c-Met, β-catenin, EGFR and c-erbB2 co-expressions are summarized in Table 1. In non-malignant gallbladder mucosal epithelial cells, signals for Cox-2, c-Met, EGFR and c-erbB2 were minimal or negative, while that for β-catenin was expressed in 80% of non-malignant specimens exclusively in the cell membrane. Some of the metaplastic cells showed positive staining for both c-erbB2 and Cox-2.
The data on the Cox-2, c-Met, β-catenin, EGFR and c-erbB2 expressions in the adenomas are summarized in Table 1. c-erbB2 and c-Met were expressed in 2 of the 7 (28%) adenoma specimens, while β-catenin was expressed in all seven adenoma specimens. Five adenomas had both nuclear and cytoplasmic staining for β-catenin, with the remaining two exhibiting predominant cytoplasmic staining. Cox-2 and c-Met were predominantly expressed in cytoplasmic and membrane localizations, while those of EGFR and c-erbB2 were present in the cancer cell membranes. Cox-2 was also present in infiltrating inflammatory and stromal cells. Twenty-two invasive cancer specimens exhibited weak membrane and moderate cytoplasmic staining for β-catenin. Nuclear localization of β-catenin was demonstrated in three invasive cancer specimens (Fig. 1). The co-expressions of Cox-2, c-Met, β-catenin and c-erbB2 were the most frequent combination in the cancer forming the invasive margin, especially in the cells invading matrix (43%). Increased immunoreactivities of Cox-2, c-Met and β-catenin in cancer cells forming invasive front were present in 61, 76 and 65% of positive specimens, respectively, compared to the central area. Significantly higher Cox-2, c-Met and β-catenin expressions were present in the cancer cells of the invasive front or of the cancer-stromal interface compared to those of the central area (Fig. 2) (Table 2). The co-expressions of Cox-2, c-Met, β-catenin, EGFR and c-erbB2 in cancer cells were present exclusively in the T2- T4 classified specimens (Table 3).
DISCUSSIONIn this study, the overexpressions of Cox-2, c-Met, β-catenin and c-erbB2 in gallbladder cancer were greater than those in the normal mucosa or adenoma, with a spatial difference in β-catenin expression found between cancer cells and normal mucosal cells. We also found the co-expressions of Cox-2, c-Met, β-catenin and c-erbB2 were the most frequent combination in cancer forming the invasive margin, especially in those cells directly invading the matrix. In addition, we demonstrated that Cox-2, c-Met and β-catenin expressions were significantly higher in the cells forming the invasive front of the cancer than those of the cancer central area; their overexpression was also associated with the depth of invasion. There is increasing evidence that Cox-2, c-Met, β-catenin, EGFR and c-erbB2 are co-expressed in cancer cells. Adenocarcinomas of the lung that overexpress EGFR and c-erbB2 have higher levels of Cox-2 than those without concomitant overexpressions of these proteins (23). Our previous study demonstrated that PGE2, produced by Cox-2, rapidly causes transactivation of EGFR, which triggered the ERK2-mitogenic signaling pathway in a colon cancer cell line (19). Furthermore, PGE2 increases the invasiveness of colon cancer cells by trans-activating c-Met and increasing the tyrosine phosphorylation of β-catenin (18). Our previous study also demonstrated that HGF triggered the activation of the Cox-2 gene in gastric epithelial cells, via the phosphorylation of c-Met (20). Pennacchietti et al. demonstrated that the expression of the Met receptor encoded by the Met protooncogene is induced by low oxygen tension, which activates a program of invasive growth (24). Low oxygen tension is usually found in the cancer invasive front interfacing the matrix. Taken together, these data support the contention that the coordinated expressions of Cox-2, c-Met and β-catenin, and their local interactions, play an important role in the growth and invasion and of human gallbladder cancer.
It has also been suggested that Cox-2 over-expression plays a critical role in the invasion and metastasis of colon (3,4), gastric (6), lung (23) and urinary bladder cancers (7). In our present study, Cox-2 was the most frequently over-expressed protein of those studied, which also showed the most frequent association with the expressions of c-Met, β-catenin and c-erbB2 in invasive gallbladder cancer. Our findings are in agreement with previous studies, which have demonstrated a close relationship between Cox-2 expression and tumor growth in gallbladder cancer (5). Taken together, our data suggest that Cox-2 is a critical factor, which interacts with c-Met, β-catenin and c-erbB2 proteins and enhances cancer invasiveness. However, our study showed no frequent overexpression of EGFR in invasive cancer compared to that of in situ carcinomas. This finding can partly be explained by previous reports that the activation of EGFR has been shown to stimulate the invasiveness only transiently, while c-Met activation causes prolonged stimulation of the invasiveness (25).
Localization of β-catenin, primarily to the apical-lateral cell membrane, signifies its role in cell adhesion, whereas cytoplasmic and nuclear accumulations suggest enhanced transcription and activation of the target genes (15). In this study, we have demonstrated that in normal mucosal cell linings of the gallbladder, β-catenin was localized to the membrane, while in invasive cancer cells it has increased cytoplasmic and nuclear localizations. This is in agreement with a previous study, which demonstrated loss of membrane expression and increased cytoplasmic or nuclear β-catenin expression in colorectal cancer cells (14,15).
ACKNOWLEDGMENTWe appreciate the assistance of Dr. Michael K. Jones for his discussion and editorial suggestions.
NotesThis work was supported by the Regional Research Centers Program of the Korean Ministry of Education & Human Resources Development through the Center for Healthcare Technology Development. References1. Ito H, Matros E, Brooks DC, Osteen RT, Zinner MJ, Swanson RS, et al. Treatment outcomes associated with surgery for gallbladder cancer: a 20-year experience. J Gastrointest Surg. 2004;8:183–190. PMID: 15036194
2. Aldridge MC, Bismuth H. Gallbladder cancer: the polyp-cancer sequence. Br J Surg. 1990;77:363–364. PMID: 2187556
3. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. PMID: 7926468
4. van Rees BP, Ristimaki A. Cyclooxygenase-2 in carcinogenesis of the gastrointestinal tract. Scand J Gastroenterol. 2001;36:897–903. PMID: 11521977
5. Asano T, Shoda J, Ueda T, Kawamoto T, Todoroki T, Shimonishi M, et al. Expressions of cyclooxygenase-2 and prostaglandin E-receptors in carcinoma of the gallbladder: crucial role of arachidonate metabolism in tumor growth and progression. Clin Cancer Res. 2002;8:1157–1167. PMID: 11948128
6. Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, et al. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876–1881. PMID: 11346869
7. Shirahama T, Arima J, Akiba S, Sakakura C. Relation between cyclooxygenase-2 expression and tumor invasiveness and patient survival in transitional cell carcinoma of the urinary bladder. Cancer. 2001;92:188–193. PMID: 11443626
8. Rozic JG, Chakraborty C, Lala PK. Cyclooxygenase inhibitors retard murine mammary tumor progression by reducing tumor cell migration, invasiveness and angiogenesis. Int J Cancer. 2001;93:497–506. PMID: 11477553
9. Prigent SA, Lemoine NR. The type 1 (EGFR-related) family of growth factor receptors and their ligands. Prog Growth Factor Res. 1992;4:1–24. PMID: 1355372
10. Tokunaga A, Onda M, Okuda T, Teramoto T, Fujita I, Mizutani T, et al. Clinical significance of epidermal growth factor (EGF), EGF receptor, and c-erbB-2 in human gastric cancer. Cancer. 1995;75(Suppl. 6):1418–1425. PMID: 7889468
11. Lee CS, Pirdas A. Epidermal growth factor receptor immunoreactivity in gallbladder and extrahepatic biliary tract tumours. Pathol Res Pract. 1995;191:1087–1091. PMID: 8822109
12. Suzuki T, Takano Y, Kakita A, Okudaira M. An immunohistochemical and molecular biological study of c-erbB-2 amplification and prognostic relevance in gallbladder cancer. Pathol Res Pract. 1993;189:283–292. PMID: 8101375
13. Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. PMID: 9407023
14. Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356–10361. PMID: 11526241
15. Wong NA, Pignatelli M. Beta-catenin--a linchpin in colorectal carcinogenesis? Am J Pathol. 2002;160:389–401. PMID: 11839557
16. Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. PMID: 6590967
17. Danilkovitch-Miagkova A, Zbar B. Dysregulation of met receptor tyrosine kinase activity in invasive tumors. J Clin Invest. 2002;109:863–867. PMID: 11927612
18. Pai R, Nakamura T, Moon WS, Tarnawski AS. Prostaglandins promote colon cancer cell invasion; signaling by cross-talk between two distinct growth factor receptors. FASEB J. 2003;17:1640–1647. PMID: 12958170
19. Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–293. PMID: 11875501
20. Jones MK, Sasaki E, Halter F, Pai R, Nakamura T, Arakawa T, et al. HGF triggers activation of the COX-2 gene in rat gastric epithelial cells: action mediated through the ERK2 signaling pathway. FASEB J. 1999;13:2186–2194. PMID: 10593866
21. Albores-Saavedra J, Henson DE, Sobin LH. WHO histologic typing of tumors of the gallbladder and extrahepatic bile ducts. International Histologic Classification of Tumors. 1991. 2nd edBerlin: Springer-Verlag.
22. Gallbladder. American Joint Committee on Cancer: AJCC Cancer staging manual. 1997. 5th edPhiladelphia, Pa: Lippincott-Raven Publishers; p. 103–108.
23. Niki T, Kohno T, Iba S, Moriya Y, Takahashi Y, Saito M, et al. Frequent co-localization of Cox-2 and laminin-5 gamma2 chain at the invasive front of early-stage lung adenocarcinomas. Am J Pathol. 2002;160:1129–1141. PMID: 11891209
24. Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. PMID: 12726861
25. Comoglio PM, Trusolino L. Invasive growth: from development to metastasis. J Clin Invest. 2002;109:857–862. PMID: 11927611
Fig. 1Representative immunostaining for EGFR, c-erbB2, Cox-2, c-Met and β-catenin in gallbladder cancer. A-B) Cancer cells showed selective membrane staining for EGFR (A, ×200, scale bar 100 µm) and c-erbB2 (B, ×400, scale bar 100 µm). Note the absence of immunoreactivity in normal mucosal cells. Immunohistochemistry for c-Met (C, ×400, scale bar 100 µm) and Cox-2 (D, ×400, scale bar 100 µm) displayed cytoplasmic and membrane positivity. E-G) β-catenin staining showed normal mucosal cells, with membrane staining (E, ×200, scale bar 100 µm), cytoplasmic immunoreactivity in cancer cells (F, ×100, scale bar 100 µm), and nuclear localization in cancer cells at the invasive front (G, ×400, scale bar 100 µm). 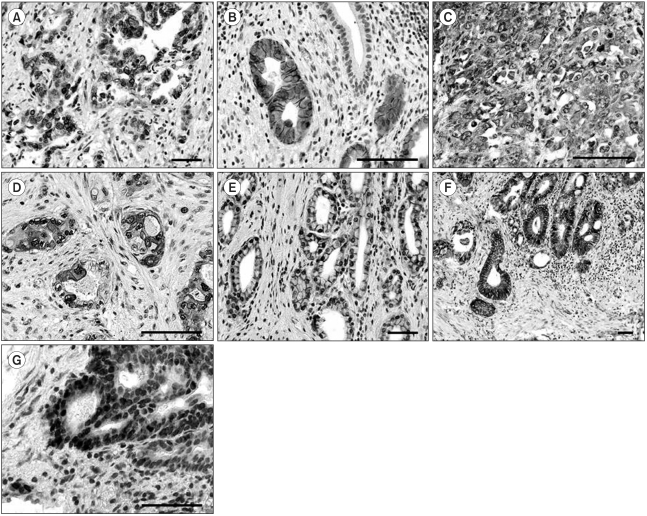
Fig. 2Comparison of the signal intensities in cancer cells between the central area and invasive front. A-B) β-catenin (A, ×100, scale bar 100µm) (B, ×100, scale bar 100µm), C-D) Cox-2 (C, ×40, scale bar 1 mm) (D, ×100, scale bar 100µm) and E-F) c-Met (E, ×40, scale bar 1 mm) (F, ×100, scale bar 100µm). Note the increased immunoreactivities of Cox-2, c-Met and β-catenin in cancer cells forming the invasive front (arrows) compared to those of cells in the central area. 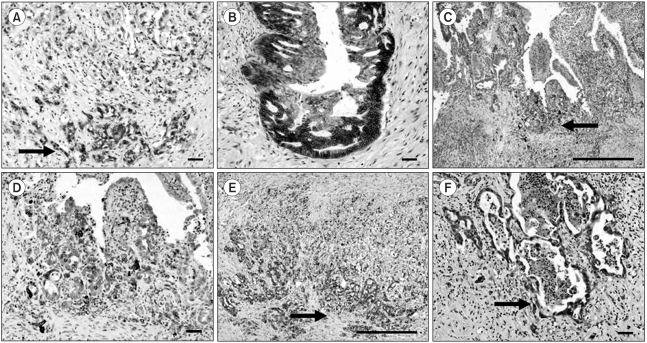
Table 1Expressions of Cox-2, c-Met, β-catenin, EGFR and c-erbB2 in invasive gallbladder cancer, carcinomas in situ and gallbladder adenomas 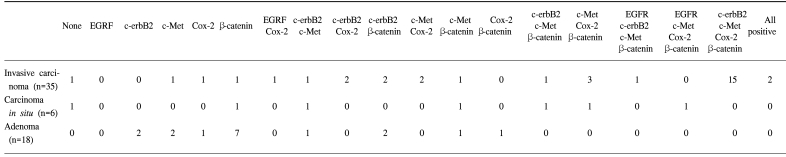
|
|
|||||||||||||||||||||||||||||||||||||||||