AbstractPurposeThe programmed death-ligand 1 (PD-L1) SP142 assay identifies patients with triple-negative breast cancer (TNBC) who are most likely to respond to the anti–PD-L1 agent atezolizumab. We aimed to compare PD-L1 (SP142) expression between primary and recurrent/metastatic TNBCs and elucidate the clinicopathological features associated with its expression.
Materials and MethodsPrimary and recurrent/metastatic TNBCs tested with PD-L1 (SP142) were collected, and clinicopathological information of these cases was obtained through a review of slides and medical records.
ResultsPD-L1 (SP142) positivity was observed in 50.9% (144/283) of primary tumors and 37.8% (31/82) of recurrent/metastatic TNBCs with a significant difference. Recurrent or metastatic sites were associated with PD-L1 positivity, with high PD-L1 positivity in the lung, breast, and soft tissues, and low positivity in the bone, skin, liver, and brain. When comparing PD-L1 expression between primary and matched recurrent/metastatic TNBCs using 55 paired samples, 20 cases (36.4%) showed discordance; 10 cases revealed positive conversion, and another 10 cases revealed negative conversion during metastatic progression. In primary TNBCs, PD-L1 expression was associated with a higher histologic grade, lower T category, pushing border, and higher tumor-infiltrating lymphocyte infiltration. In survival analyses, PD-L1 positivity, especially high positivity, was found to be associated with favorable prognosis of patients.
ConclusionPD-L1 (SP142) expression was lower in recurrent/metastatic TNBCs, and substantial cases showed discordance in its expression between primary and recurrent/metastatic sites, suggesting that multiple sites may need to be tested for PD-L1 (SP142) when considering atezolizumab therapy. PD-L1 (SP142)–positive TNBCs seems to be associated with favorable clinical outcomes.
IntroductionTriple-negative breast cancers (TNBCs), which are defined as breast cancers that are negative for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2), are known to be aggressive and show poor clinical outcome [1]. As patients with TNBC cannot benefit from endocrine therapy or HER2-targeted therapy, other therapeutic agents, including poly adenosine diphosphate-ribose polymerase inhibitors, such as olaparib [2] and talazoparib [3], and immunotherapy have been developed in attempts to treat TNBC patients.
Generally, breast cancer is known to be a less immunogenic tumor with low mutational burden [4]. However, the mutational load is higher in TNBCs than in non-TNBCs [5]. Programmed death-ligand 1 (PD-L1) expression is also higher, and more tumor-infiltrating lymphocytes (TILs) are found in TNBCs [6,7]. Tumor mutational burden, TILs, and PD-L1 expression are predictive biomarkers of immune checkpoint inhibitor therapy; thus, immunotherapy has been considered in TNBCs [8].
The programmed death 1 (PD-1) receptor is an immuneinhibitory receptor expressed in immune cells (ICs), including activated T cells, B cells, and myeloid cells, and acts by binding to PD-L1 within the tumor immune microenvironment [9,10]. Pembrolizumab (a PD-1 inhibitor) and atezolizumab (a PD-L1 inhibitor) prevent the interaction between PD-1 and PD-L1, resulting in the reversal of T cell suppression. They are immune checkpoint inhibitors that have been approved for treatment of locally advanced or metastatic TNBCs through Keynote-355 [11] and IMpassion130 trials [12]. These trials have also proved the value of PD-L1 as a predictive biomarker for the efficacy of pembrolizumab or atezolizumab in locally advanced or metastatic TNBC patients, although different antibodies and scoring systems are required. For pembrolizumab, PD-L1 IHC 22C3 PharmDx assay is used and combined positive score of 10 or more is considered positive. On the other hand, VENTANA PD-L1 (SP142) assay is used for atezolizumab and an IC score of 1% is used as cutoff.
For the samples to be tested, both primary and metastatic tumor samples were used to evaluate the PD-L1 status. However, the IMpassion130 trial and some other studies have shown that PD-L1 IC positivity is higher in primary tumors than in metastatic tumors. In addition, PD-L1 positivity in metastatic TNBCs has been found to vary depending on the site of metastasis [12-14]. If these findings are consistent, they can have important clinical implications in the selection of samples to be tested. Moreover, only a few studies have compared PD-L1 expression between primary TNBCs and their matched metastases. Most of them included not only TNBCs but also other subtypes, and even those that included only TNBCs evaluated a limited number (up to 45 cases) of paired samples [13,15-18].
Thus, in this study, we aimed to compare PD-L1 (SP142) expression between primary and recurrent/metastatic TNBCs and evaluate PD-L1 positivity by the site of recurrence or metastasis using a large number of cases. In particular, we compared PD-L1 (SP142) expression between paired primary and metastatic TNBC samples. We also attempted to elucidate the clinicopathological features associated with PD-L1 (SP142) expression and to determine the prognostic value of PD-L1 (SP142) expression in TNBCs.
Materials and Methods1. Patients and samplesPrimary and recurrent/metastatic TNBCs tested for PD-L1 (SP142) at Seoul National University Bundang Hospital between 2019 and 2022 were collected. In total, 310 patients (365 samples) were included in this study. Two hundred eighty-three samples were from primary TNBCs, and 82 samples were from recurrent or metastatic TNBCs. One hundred and fifty-seven samples were from surgical resection specimens, and 208 samples were from core needle biopsy specimens. Fifty-five primary and matched recurrent/metastatic samples were used for the paired comparisons.
2. Clinicopathological informationClinicopathological information of the selected cases was obtained through a review of the slides and medical records. The following information was collected: age at diagnosis, sex, histologic type, histologic grade, primary tumor size, T category, N category, lymphovascular invasion, perineural invasion, tumor border, TILs, and immunohistochemical results for p53, cytokeratin 5/6, and epidermal growth factor receptor, site of recurrence or metastasis and type of systemic therapy. In patients who received neoadjuvant chemotherapy (NAC), the primary tumor size was measured based on imaging studies prior to treatment, and clinical staging for T and N categories was applied. In patients who underwent immediate surgery without NAC, the microscopic size of the tumor and pathological staging for T and N categories were used. TIL were scored based on the tutorial and reference images created by the International Working Group for TIL in breast cancer, and were categorized as < 10%, ≥ 10% and < 50%, and ≥ 50%. Clinicopathological characteristics of the patients included in this study are summarized in Table 1.
Clinical follow-up data were also collected for each patient. In patients who received NAC before surgery, recurrence-free survival was defined as the period from the start of NAC to the date of clinical detection of recurrence, and cancer-specific survival was defined as the period from the start of NAC to the date of death due to TNBC. The date of surgery was used for patients who had undergone upfront surgery. In cases where recurrence or death did not occur, the follow-up time was calculated from the date of the start of NAC or surgery to the date of the last event-free follow-up.
3. Immunohistochemical staining for PD-L1Immunohistochemical (IHC) staining for PD-L1 (SP142) was performed on 4-μm-thick sections from formalin-fixed paraffin-embedded tissue blocks using the OptiView DAB IHC detection kit (Ventana Medical Systems, Tucson, AZ) and OptiView Amplification Kit (Ventana Medical Systems) on a BenchMark ULTRA platform (Ventana Medical Systems) according to the manufacturer’s instructions.
When only biopsy specimens were available, IHC was performed on the biopsy sections. For surgically resected samples, one representative tumor block was chosen for PD-L1 staining. To exclude the effect of NAC on the expression of PD-L1, pre-NAC biopsy specimens were used in patients who underwent NAC.
4. Interpretation of PD-L1 (SP142) stainingInterpretation of the PD-L1 SP142 assay was based on Ventana’s interpretation guide for TNBC. PD-L1 staining of tumor-infiltrating ICs was scored. As stated in the interpretation guide, lymphocytes, macrophages, and cells with dendritic or reticular morphology in the intratumoral and contiguous peritumoral stroma are regarded as tumor-infiltrating ICs. The IC score was calculated as the proportion of the tumor area occupied by PD-L1–stained ICs of any intensity. A specimen was considered positive for PD-L1 (SP142) if it showed a ≥ 1% IC score. According to the IMpassion130 trial [12], specimens with ≥ 5% IC scores were considered to have high PD-L1 expression.
5. Statistical analysesStatistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Corp., Armonk, NY). The chi-square test or Fisher’s exact test was used to compare PD-L1 positivity between primary and recurrent/metastatic tumors or between different recurrent or metastatic sites, and to evaluate the clinicopathological features of tumors associated with PD-L1 expression. The difference in TIL levels in recurrent/metastatic tumors was analyzed by Kruskal-Wallis test among three groups, and by Mann-Whitney U test between two groups. Corrections for multiple testing were made by Bonferroni method, and adjusted p-values were calculated. The Wilcoxon signed-rank test was used to evaluate the change in PD-L1 expression in paired primary and recurrent/metastatic samples.
Recurrence-free survival and cancer-specific survival were calculated using the survival analysis. Survival curves were drawn using the Kaplan-Meier method, and p-values were calculated using the log-rank test. The Cox proportional hazards model was used for multivariate analysis using the backward stepwise selection method. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for each variable. All p-values were two-sided, and p-values less than 0.05 were considered statistically significant.
Results1. Comparison of PD-L1 (SP142) expression between primary and recurrent/metastatic TNBCsOverall, 175 of the 365 cases (47.9%) were positive for PD-L1 (SP142) and 120 of the 175 PD-L1–positive cases (68.6%) showed high PD-L1 expression. Among 283 primary tumors, 144 (50.9%) were PD-L1 positive. In contrast, of the 82 recurrent/metastatic cases, 31 (37.8%) were positive for PD-L1; four (57.1%) of the seven recurrent tumors and 27 of the 75 metastatic tumors (36.0%) were PD-L1 positive. Primary TNBCs showed higher PD-L1 positivity than recurrent/metastatic TNBCs (p=0.037, chi-square test) (Fig. 1).
We analyzed the specimen type of primary and recurrent/metastatic samples to check if the difference in PD-L1 expression was related to the difference in specimen type: 173 out of 283 primary samples (61.1%) and 35 out of 82 recurrent/metastatic samples (42.7%) were biopsied samples.
2. PD-L1 (SP142) expression and TIL levels according to recurrent/metastatic sitesNext, PD-L1 positivity and TIL levels were evaluated according to the site of recurrence or metastasis (Table 2). Recurrent or metastatic sites were associated with PD-L1 positivity. PD-L1 positivity was high in the lung (58.3%), breast (57.1%), and soft tissue (57.1%), and intermediate in the lymph nodes (31.8%). Bone (0%), skin (0%), liver (11.1%), and brain (16.7%) were the sites with the lowest PD-L1 positivity. PD-L1 positivity showed a difference between the three groups which were classified based on PD-L1 positivity (p=0.001).
We also examined TIL levels in the 82 recurrent or metastatic samples. TIL levels were variable but showed a difference according to recurrent or metastatic sites. Median TIL levels were high in the lung (30%), breast (10%) and soft tissue (10%), intermediate in the lymph node (5%), and low in the bone, brain, liver, and skin (1% in all) (Table 2). TIL levels were different among the three groups (high, intermediate and low TIL groups; p=0.002, Kruskal-Wallis test), with significant differences between intermediate and low TIL groups (adjusted p=0.045, Mann-Whitney U test), as well as between high and low TIL groups (adjusted p < 0.001, MannWhitney U test). Collectively, TIL levels (< 10% vs. ≥ 10%) showed a close relationship with PD-L1 positivity in these recurrent or metastatic samples (9.1% vs. 71.1%; p < 0.001).
3. Paired comparison of PD-L1 (SP142) expression in the primary and matched recurrent/metastatic TNBCsA paired comparison of PD-L1 expression between primary and matched recurrent/metastatic TNBC was performed in 55 cases. When the level of PD-L1 expression was compared as a continuous variable, no significant difference was found (p=0.560, Wilcoxon signed-rank test).
Of the 55 paired samples, 22 (40.0%) were PD-L1 positive in both the primary and recurrent/metastatic tumors. However, 10 cases that were initially PD-L1 positive showed negative conversion in recurrent/metastatic tumors, and another 10 cases that were initially PD-L1 negative showed positive conversion (Fig. 2). As a result, 20 cases (36.4%) showed discordant results between the primary and recurrent/metastatic tumors. Negatively converted cases were distributed as follows: brain (n=1), lung/pleura (n=5), lymph node (n=1), and skin (n=3). Positively converted cases were distributed as follows: breast (n=3), liver (n=1), lung/pleura (n=5), and soft tissue (n=1) (Table 3).
In the paired analysis, twelve cases showed high PD-L1 expression in the primary tumor and five (41.7%) of them showed negative conversion in metastasis. The remaining seven cases were positive for PD-L1 in the metastatic sites, five of which still showed high PD-L1 expression.
Among the paired 55 cases, five presented as synchronous metastasis and the remaining 50 presented as metachronous metastasis. Two cases (2/5, 40.0%) in the synchronously metastasized group and 18 cases (18/50, 36.0%) in the metachronously metastasized group showed discordant PD-L1 status after metastatic progression.
4. Clinicopathological characteristics associated with PD-L1 (SP142) expressionUsing 283 primary TNBC cases, the clinicopathological features associated with PD-L1 expression were evaluated (Table 4). PD-L1 positivity was associated with invasive carcinoma of no special type (invasive carcinoma of no special type vs. special types of invasive carcinoma, p=0.012 by chi-square test), higher histologic grade (II vs. III, p < 0.001), lower T category (T1-2 vs. T3-4, p=0.004), pushing border (p=0.001), and higher TIL infiltration (< 10% vs. ≥ 10%, p < 0.001).
5. Prognostic significance of PD-L1 expression in primary TNBCsAmong the 283 primary TNBCs, 264 were included in the survival analyses. Of the 19 excluded cases, 17 presented with synchronous metastasis, one could not be resected due to deterioration of the patient’s condition, and another was non-operable at the time of presentation. In the 264 cases, median follow-up was 2.3 years (range, 0.4 to 18.2 years). Of these, 156 were treated with NAC before surgery. Among them, 27 cases presented with recurrence afterwards, and four patients died. The remaining 108 patients underwent upfront surgery without NAC, 34 of whom experienced recurrence and six of whom died.
The Kaplan-Meier survival curve for recurrence-free survival showed increased survival time in the PD-L1–positive group than in the PD-L1–negative group (p=0.049 by log-rank test) (Fig. 3A). Especially, PD-L1 high tumors (≥ 5% IC) showed favorable survival compared to non–PD-L1 high tumors (p=0.005, log-rank test) (Fig. 3B). The survival curve for cancer-specific survival tended to show increased survival in the PD-L1 positive group, but the difference was not statistically significant (p=0.140, log-rank test).
In univariate analyses of recurrence-free survival, age, T category, N category, lymphovascular invasion, perineural invasion, TIL, and PD-L1 high expression were found to be statistically significant prognostic factors (Table 5). In multivariate analyses of recurrence-free survival, lymphovascular invasion (HR, 2.523; 95% CI, 1.431 to 4.448; p=0.001) and PD-L1 high expression (HR, 0.422; 95% CI, 0.205 to 0.872; p=0.020) remained independent prognostic factors (Table 5).
DiscussionOur study demonstrated that primary TNBCs were more likely to be PD-L1 (SP142)–positive than recurrent/metastatic TNBCs. In this study, 50.9% of primary TNBCs and 36.0% of metastatic TNBCs were PD-L1–positive. This result is in concordance with previous studies that showed higher PD-L1 IC positivity in primary TNBCs (44.0-62.0%) compared to metastatic ones (31.0-42.2%) [12-14]. PD-L1 positivity in TNBC has been reported to be associated with increased stromal TILs [13]. Previous studies have shown that there are some differences in the immune microenvironment in primary and metastatic breast cancer with lower TIL in metastatic lesions [19-21], which might have caused the difference.
It is well known that PD-L1 expression in tumors can be spatially heterogeneous, and it has been reported that PD-L1 (SP142) IC positivity in surgical samples is statistically higher than in biopsied samples [22]. When we analyzed the specimen type of primary and recurrent/metastatic samples, primary samples consisted more of biopsied samples compared to recurrent/metastatic samples (61.1% vs. 42.7%). Therefore, lower PD-L1 positivity in metastatic samples might not be related to the specimen type.
Another study using 30 matched paired primary and metastatic TNBC samples suggested that the time of sample collection may influence PD-L1 positivity, with PD-L1 status agreement being higher in synchronously collected cases (80%) than in asynchronously collected cases (75%) [17]. Of the 55 paired samples, discordance in PD-L1 status was 40% in synchronous metastases and 36% in metachronous metastases, suggesting that the timing of sample collection may not have an influence on changes in PD-L1 status.
In the present study, recurrence or metastatic sites was associated with PD-L1 positivity, with high PD-L1 positivity in breast, lung, and soft tissue, and low PD-L1 positivity in bone, brain, liver, and skin. Although PD-L1 expression in other sites varied among studies, low positivity in the liver has consistently been noted, ranging from 13% to 17.4% [12-14]. In our study, similar results were found, with 11.1% of liver samples being PD-L1 positive. We wondered whether the varying distribution of TILs in metastatic TNBC across organs could explain the observed differences in PD-L1 expression. In one relevant study on TNBCs, TIL levels were found to be highest in the lung and lowest in the skin, although the difference was not statistically significant [23], and in another study on breast cancer including other subtypes, brain metastases had a lower amount of TILs compared to metastatic breast cancers of other locations [20]. Our study showed similar results to the previous studies, with high level of TILs in the lung, and low level of TILs in the bone, brain, liver, and skin. Thus, it is possible that different PD-L1 positivity according to metastatic sites is related to TIL infiltration levels in the metastatic sites.
Hoda et al. [13] have shown a discordance in PD-L1 (SP142) expression in four out of eight paired primary and second site TNBCs, although this result was limited due to the small number of cases. In their study, all discordant cases showed negative conversion. However, it should be noted that out of these four cases, three were from the liver, and one was from bone, both of which sites are known to show low positivity for PD-L1 [13]. In one systemic review on breast cancer, discordance in PD-L1 expression in paired primary and metastatic tumors was reported to be 39.5% and the direction of change was more commonly from PD-L1 positive primary tumor to PD-L1 negative metastasis [24]. Recently, Miyakoshi et al. [18] reported the concordance of PD-L1 status between primary tumors and metastases in 160 patients with TNBC, including 45 paired samples. In their study, 16 of the 45 paired samples (35.6%) showed discordance for PD-L1 (SP142) with seven cases of positive conversion and nine cases of negative conversion in metastasis [18], which was consistent with the findings of our study. We used 55 paired samples for matched comparison, and there was a discordance of PD-L1 (SP142) expression in 20 out of 55 cases (36.4%). In addition to the 10 negatively converted cases, there were 10 positively converted cases. These results suggested that PD-L1 status in primary TNBC samples cannot reliably predict its status in recurrent or metastatic samples. We also evaluated changes in PD-L1 status according to the recurrent/metastatic sites, but the number of cases was too small to observe a trend of change. However, it is worth noting that more than half of the recurrent samples in the breast were positively converted, and all three metastatic samples in the skin were negatively converted. The type of recurrent or metastatic site and its immune microenvironment appear to be related to PD-L1 status in recurrent or metastatic tumors.
In IMpassion130 trial, combining atezolizumab in PD-L1 IC-positive TNBC cases showed a clinical benefit regardless of whether the sample was from primary or metastatic tumor [12]. Taking into account these findings, the question of which tissue should be used for evaluation of PD-L1 (SP142) expression arises. One expert committee recommended that the primary tumor should be primarily evaluated for PD-L1 expression and if the result is negative, at least one metastatic tumor should be tested [25]. They also mentioned the priority of choosing the site for PD-L1 testing should be given according to the positivity [25]. Based on the post-hoc analysis of the IMpassion130 trial in which PD-L1 positivity was the highest in the lymph nodes (51%), they designated the lymph node as the sample of choice for PD-L1 evaluation in metastasis [25]. However, only 31.8% of the lymph node samples were positive for PD-L1 in our study. This may be due to the difference in delimiting the tumor area and stroma in the lymph nodes, as they are rich in ICs. Despite this difference, we agree that multiple sites of the tumor may need to be tested to maximize the patients’ chances of receiving immunotherapy. However, inhomogeneous PD-L1 expression at different metastatic sites may be associated with varied responses to immune checkpoint inhibitor, and further studies are needed.
In the present study, PD-L1 expression was associated with invasive carcinoma of no special type, higher histologic grade, lower T category, pushing border, and higher percentage of TILs. In addition, high PD-L1 expression is associated with longer recurrence-free survival. The prognostic value of PD-L1 expression in breast cancer patients remains controversial. Some studies have reported that PD-L1 expression in non-metastatic TNBC leads to better recurrence-free survival and overall survival, while another study showed that its expression in TNBC was an independent poor prognostic factor for overall survival [26-28]. The reason why PD-L1 expression was related to better prognosis in this study remains unclear. One possible explanation is that PD-L1 expression was related to some favorable features in our study, such as lower T category and higher percentage of TIL. In particular, TIL are potential prognostic factors in TNBC, as a high percentage of TILs is associated with a better response to NAC and improved survival in early-stage TNBCs [29,30].
In summary, our study showed that PD-L1 (SP142) expression is lower in recurrent/metastatic TNBCs and that there is a discordance in PD-L1 (SP142) expression in primary and recurrent/metastatic TNBCs, suggesting that multiple sites may need to be tested for PD-L1 when atezolizumab therapy is being considered. PD-L1 (SP142) positivity, especially high PD-L1 positivity, appears to be associated with favorable clinical outcomes in TNBCs.
NotesEthical Statement This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-2111-723303). The need for informed consent was waived as this study was in retrospective nature. All data were anonymized and de-identified before analysis. Author Contributions Conceived and designed the analysis: Han EK, Park SY. Collected the data: Han EK, Woo JW. Contributed data or analysis tools: Han EK, Suh KJ, Kim SH, Kim JH, Park SY. Performed the analysis: Han EK, Park SY. Wrote the paper: Han EK, Park SY. Supervision: Suh KJ, Kim SH, Kim JH, Park SY. AcknowledgmentsThis study was supported by a grant from the National Research Foundation of Korea (NRF)’s Basic Science Research Program to Park SY by the Ministry of Science, ICT and Future Planning (Grant No. 2022R1F1A1065468).
Fig. 1.Comparison of programmed death-ligand 1 (PD-L1) (SP142) expression between primary and recurrent/metastatic triple-negative breast cancers. Of a total of 365 cases, 175 (47.9%) were positive for PD-L1 (SP142). Among the 283 primary tumors, 144 (50.9%) were PD-L1–positive, whereas four of the seven recurrent tumors (57.1%) and 27 of the 75 metastatic tumors (36.0%) were PD-L1–positive. 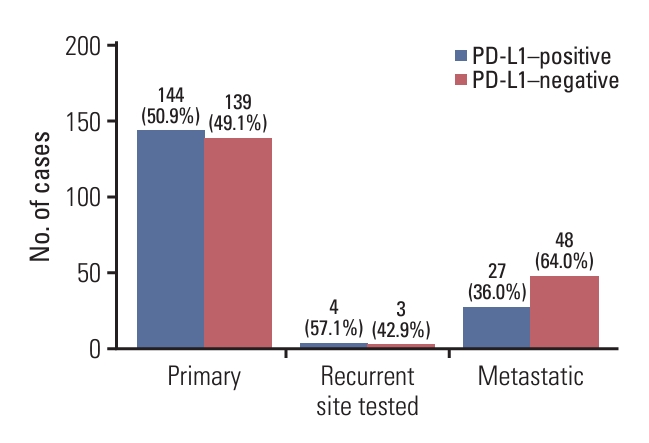
Fig. 2.Representative cases of positive and negative conversion of programmed death-ligand 1 (PD-L1) (SP142) expression in the metastatic sites. (A, B) A case with negative conversion. Primary tumor (A) shows PD-L1 positivity, while metastatic tumor to the skin (B) shows PD-L1 negativity. (C, D) A case showing positive conversion. Primary tumor (C) is negative for PD-L1 and metastatic tumor in the chest wall (D) is positive for PD-L1. 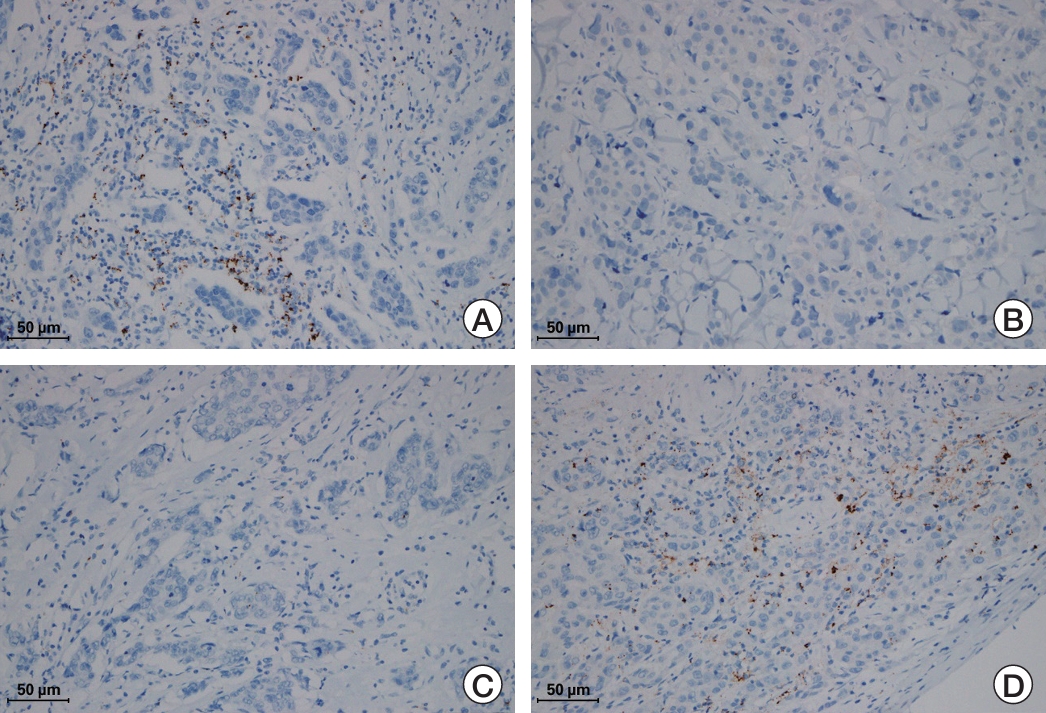
Fig. 3.Kaplan-Meier survival curve for recurrence-free survival according to programmed death-ligand 1 (PD-L1) (SP142) status. (A) Kaplan-Meier survival curve shows increased survival time in the PD-L1–positive group compared to PD-L1–negative group (p=0.049 by log-rank test). (B) PD-L1 high tumors show better survival compared to non–PD-L1 high tumors (p=0.005 by log-rank test). 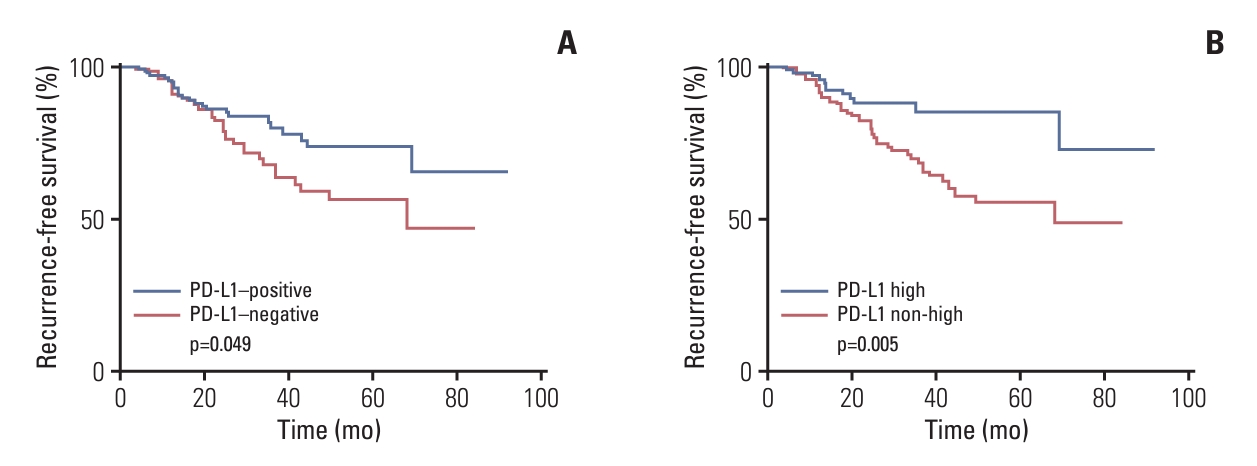
Table 1.Clinicopathological characteristics of the patients Table 2.PD-L1 (SP142) positivity and TIL levels according to recurrent or metastatic sites Table 3.Change of PD-L1 (SP142) status by recurrent or metastatic site in paired comparison of primary and matched recurrent/metastatic samples Table 4.Clinicopathological characteristics associated with PD-L1 (SP142) positivity Values are presented as number (%). p-values are calculated by chi-square test or Fisher’s exact test. CK, cytokeratin; EGFR, epidermal growth factor receptor; IC-NST, invasive carcinoma of no special type; IC-ST, special types of invasive carcinoma; PD-L1, programmed death-ligand 1; TIL, tumor-infiltrating lymphocyte. Table 5.Univariate and multivariate analyses of recurrence-free survival p-values are calculated by Cox proportional hazards model using the backward stepwise selection method. CI, confidence interval; HR, hazard ratio; IC, immune cell; IC-NST, invasive carcinoma of no special type; IC-ST, special types of invasive carcinoma; LVI, lymphovascular invasion; PD-L1, programmed death-ligand 1; PNI, perineural invasion; TIL, tumor-infiltrating lymphocyte. References1. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74.
2. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33.
3. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–63.
4. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8.
5. Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29:241–50.
6. Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–70.
7. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7.
8. Tan Q, Yin S, Zhou D, Chi Y, Man X, Li H. Potential predictive and prognostic value of biomarkers related to immune checkpoint inhibitor therapy of triple-negative breast cancer. Front Oncol. 2022;12:779786.
9. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34.
11. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–28.
12. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–21.
13. Hoda RS, Brogi E, Dos Anjos CH, Grabenstetter A, Ventura K, Patil S, et al. Clinical and pathologic features associated with PD-L1 (SP142) expression in stromal tumor-infiltrating immune cells of triple-negative breast carcinoma. Mod Pathol. 2020;33:2221–32.
14. Rozenblit M, Huang R, Danziger N, Hegde P, Alexander B, Ramkissoon S, et al. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J Immunother Cancer. 2020;8:e001558
15. Manson QF, Schrijver W, Ter Hoeve ND, Moelans CB, van Diest PJ. Frequent discordance in PD-1 and PD-L1 expression between primary breast tumors and their matched distant metastases. Clin Exp Metastasis. 2019;36:29–37.
16. Tawfik O, Kimler BF, Karnik T, Shehata P. Clinicopathological correlation of PD-L1 expression in primary and metastatic breast cancer and infiltrating immune cells. Hum Pathol. 2018;80:170–8.
17. Li Y, Vennapusa B, Chang CW, Tran D, Nakamura R, Sumiyoshi T, et al. Prevalence study of PD-L1 SP142 assay in metastatic triple-negative breast cancer. Appl Immunohistochem Mol Morphol. 2021;29:258–64.
18. Miyakoshi J, Yazaki S, Shimoi T, Onishi M, Saito A, Kita S, et al. Discordance in PD-L1 expression using 22C3 and SP142 assays between primary and metastatic triple-negative breast cancer. Virchows Arch. 2023;483:855–63.
19. Ogiya R, Niikura N, Kumaki N, Bianchini G, Kitano S, Iwamoto T, et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016;107:1730–5.
20. Cimino-Mathews A, Ye X, Meeker A, Argani P, Emens LA. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol. 2013;44:2055–63.
21. Sobottka B, Pestalozzi B, Fink D, Moch H, Varga Z. Similar lymphocytic infiltration pattern in primary breast cancer and their corresponding distant metastases. Oncoimmunology. 2016;5:e1153208
22. Baek SH, Kim JH, Bae SJ, Ji JH, Lee Y, Jeong J, et al. SP142 PD-L1 assays in multiple samples from the same patients with early or advanced triple-negative breast cancer. Cancers (Basel). 2022;14:3042.
23. Dieci MV, Tsvetkova V, Orvieto E, Piacentini F, Ficarra G, Griguolo G, et al. Immune characterization of breast cancer metastases: prognostic implications. Breast Cancer Res. 2018;20:62.
24. Boman C, Zerdes I, Martensson K, Bergh J, Foukakis T, Valachis A, et al. Discordance of PD-L1 status between primary and metastatic breast cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2021;99:102257.
25. Peg V, Lopez-Garcia MA, Comerma L, Peiro G, Garcia-Caballero T, Lopez AC, et al. PD-L1 testing based on the SP142 antibody in metastatic triple-negative breast cancer: summary of an expert round-table discussion. Future Oncol. 2021;17:1209–18.
26. Ghosh J, Chatterjee M, Ganguly S, Datta A, Biswas B, Mukherjee G, et al. PDL1 expression and its correlation with outcomes in non-metastatic triple-negative breast cancer (TNBC). Ecancermedicalscience. 2021;15:1217.
27. Wang X, Liu Y. PD-L1 expression in tumor infiltrated lymphocytes predicts survival in triple-negative breast cancer. Pathol Res Pract. 2020;216:152802.
28. Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69:25–34.
|
|
||||||||||||||||||||||||||||||||||||||||