AbstractPurposeSmall cell carcinoma of the genitourinary tract (GU SCC) is a rare disease with a poor prognosis. There are only limited treatment options due to insufficient understanding of the disease. In this study, we analyzed the clinical outcomes of patients with GU SCC and their association with the tumor immune phenotype.
Materials and MethodsPatients diagnosed with GU SCC were included. Survival outcomes according to the primary location (prostate and non-prostate) and stages (limited disease [LD] and extensive disease [ED]) were analyzed. We performed multiplex immunohistochemistry (IHC) in non-prostate SCC patients and analyzed the immune cell population.
ResultsA total of 77 patients were included in this study. Their median age was 71 years, 67 patients (87.0%) were male, and 48 patients (62.3%) had non-prostate SCC. All patients with ED (n=31, 40.3%) received etoposide plus platinum (EP) as initial treatment and median overall survival (OS) was 9.7 months (95% confidence interval [CI], 7.1 to 18.6). Patients with LD (n=46, 59.7%) received EP followed by radiotherapy or surgery, and 24-months OS rate was 63.6% (95% CI, 49.9 to 81.0). The multiplex IHC analysis of 21 patients with non-prostate SCC showed that patients with a higher density of programmed death-ligand 1–expressing CD68+CD206+ M2-like macrophages had significantly worse OS outcomes with an adjusted hazards ratio of 4.17 (95% CI, 1.25 to 14.29; adjusted p=0.02).
IntroductionExtrapulmonary small cell carcinoma (SCC) is a rare and aggressive malignancy with a dismal prognosis. The genitourinary tract is one of the more common primary sites of the disease [1]. In prostate cancer, the incidence of de novo SCC is approximately 1%, while the incidence of small cell transformation of castration-resistant prostate adenocarcinoma is reported to be as high as 30% throughout the disease trajectory [2]. In bladder cancer, SCC accounts for less than 1% of primary bladder malignancies and the majority of these patients present with initially advanced disease [3].
Multimodal treatments, including surgery or externalbeam radiotherapy (EBRT) with perioperative chemotherapy, are used for patients with localized disease. Patients with distant metastasis are treated with etoposide plus platinum-based doublet chemotherapy. However, most patients experience disease progression on or immediately after primary treatment, and survival outcomes are poor, with a median of 7-13 months reported among patients with initially metastatic bladder SCC [1,3-5].
For patients with small cell lung cancer (SCLC) extensive disease, the phase 3 IMpower 133 trial and CASPIAN trial have demonstrated a significant survival benefit of adding atezolizumab or durvalumab, programmed death-ligand 1 (PD-L1) inhibitors, to etoposide plus platinum [6,7]. This suggests a potential role of immune checkpoint inhibitors in treating patients with genitourinary (GU) SCC, considering the similarities between SCLC and GU SCC, including their aggressive clinical behavior and histopathologic characteristics [8]. Also, several studies showed that primary bladder SCC has a high tumor mutational burden, which is a predictor of a response to immune checkpoint inhibitors [9-11]. However, there is insufficient evidence to support the conduction of clinical trials to evaluate the benefit of immune checkpoint inhibitors for GU SCC. Further studies to evaluate the immunologic and molecular characteristics of GU SCC are needed. Currently, there are only a few studies available.
There are limited treatment options for patients with GU SCC owing to its low incidence, and its clinical characteristics are not fully understood. In the present study, we evaluated the clinical characteristics and outcomes of patients diagnosed with GU SCC. We also investigated the immune microenvironment of the patients diagnosed with primary non-prostate (NP) GU SCC and analyzed its association with survival outcomes.
Materials and Methods1. Patients and clinical outcomesPatients with histologic diagnosis of SCC from the urinary tract or the prostate, including patients with mixed urothelial carcinoma or prostate adenocarcinoma component, respectively, from July 2009 to October 2020 at Asan Medical Center, Seoul, Korea, were included. Clinical data, including demographics, histopathology, baseline Eastern Cooperative Oncology Group (ECOG) performance status, treatment, and outcomes were extracted from the electronic medical records. The initial clinical stages of the disease were classified into two categories: limited disease (LD) if the disease was localized to an area and feasible for potentially curative resection or can be included in one radiation port to receive definitive EBRT; and extensive disease (ED) if the disease had distant metastasis and a potentially curative approach was not feasible [12]. Patients who did not have a component of SCC at the time of initial diagnosis, but confirmed to have SCC component from subsequent biopsy specimen after progression to prior treatment were determined to have transdifferentiation. Survival outcomes of the patients according to the initial stage and primary tumor locations were analyzed. Among patients with LD, survival outcomes were compared according to the local treatment modality (surgery vs. EBRT). We also performed prognostic factors analysis to evaluate potential clinical variables associated with survival.
2. Immunologic featuresAmong patients with NP GU SCC, 21 patients with tumor specimens available for multiplex immunohistochemistry (IHC) were subjected to tumor immune profiling. Multiplex IHC was performed with antibodies against CD8 (1:300, MCA1817, Bio-Rad, Hercules, CA), CD103 (1:500, ab129202, Abcam, Cambridge, UK), CD137 (1:100, 34594, Cell Signaling Technology, Danvers, MA), CD4 (1:200, ab133616, Abcam), Foxp3 (1:100, ab20034, Abcam), CD20 (1:100, ab9475, Abcam), CD68 (1:500, ab192847, Abcam), CD206 (1:500, NBP1-90020, Novus, Littleton, CO), CD11c (1:100, ab52632, Abcam), MHC-II (1:300, ab7856, Abcam), and PD-L1 (1:300, 13684S, Cell Signaling Technology).
Multiplex-stained slides were scanned using the Vectra Polaris Automated Quantitative Pathology Imaging System (Akoya Biosciences, Marlborough, MA) at 20× magnification. A representative image for training was selected in Phenochart (Akoya Biosciences), and an algorithm was created in the inForm Image Analysis software (Akoya Biosciences). Multispectral images were unmixed using the spectral library in inForm software, and tumor tissue was segmented according to the presence or absence of cytokeratin antibody expression. Based on DAPI staining, each single cell was segmented, and phenotyping was performed according to the expression compartment and intensity of each marker. For each slide, up to 10 regions of interest (ROIs) that represented the tumor microenvironment of the sample were selected. The ROIs were analyzed, and the same algorithm created in this way was applied for batch-running. The exported data were consolidated and analyzed in R software using the phenoptr (Akoya Biosciences) and phenoptrReport (Akoya Biosciences) packages. The immune cell populations with staining for each subset, including CD8+ T cells, CD4+Foxp3-helper T cells, CD4+Foxp3+ regulatory T cells, CD20+ B cells, CD68+CD206– macrophages, CD68+CD206+ macrophages, and CD11c+MHC-II+ dendritic cells (DC) were estimated. Also, PD-L1 expressing macrophages and DCs were estimated. Immune cell density in the tumor microenvironment according to stage (LD vs. ED) was compared. In addition, the association of the immune cell density with survival outcomes was analyzed.
3. Statistical analysisThe baseline characteristics were analyzed with a descriptive method. Categorical variables were compared using Fisher’s exact test or the chi-square test as appropriate. Continuous variables were compared by the Mann-Whitney U test. Survival curves were estimated by Kaplan-Meier methods and compared using log-rank tests. Progression-free survival (PFS) was defined as the time from the initiation of first-line treatment for the GU SCC, either induction chemotherapy prior to EBRT, neoadjuvant chemotherapy prior to surgery for patients with LD, or palliative chemotherapy for patients with ED, to the confirmation of objective disease progression by Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 or any cause of death, whichever came first. Overall survival (OS) was defined as the time from the initiation of the first-line treatment to any cause of death. Postprogression survival was defined as the time from the confirmation of disease progression while on first-line treatment to any cause of death. A Cox proportional hazards model was used to estimate the hazards ratio, and variables with a p-value of less than 0.10 were included in the multivariable analysis. A two-sided p-value of less than 0.05 was defined as statistically significant. All statistical analyses were performed using R ver. 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results1. Study populationA total of 77 patients diagnosed with SCC of the genitourinary tract were included in this study. Their median age was 71 years (interquartile range, 66 to 77 years), 67 patients (87.0%) were male, 48 patients (62.3%) had NP GU SCC, and 46 patients (59.7%) had LD at initial diagnosis (Table 1). Of the 48 patients with NP GU SCC, 38 patients (79.2%) had bladder as the primary origin, seven patients (14.6%) had a ureter origin, and three patients (6.2%) had a kidney origin. Seventy-two patients (93.5%) received etoposide plus platinum doublet as first-line treatment, including both neoadjuvant or induction and palliative, while five patients (6.5%) were lost to follow-up or did not receive any treatment due to poor performance or the patients’ refusal. Among the 72 patients who received first-line etoposide plus platinum doublet, 33 patients received carboplatin (45.8%), and 39 patients (54.2%) received cisplatin. Among patients with LD (n=46), more patients received cisplatin (n=27) than carboplatin (n=16), while more patients received carboplatin (n=17) than cisplatin (n=12) among those with ED (n=31).
Nineteen patients (24.7%) had transdifferentiation to SCC, while the other 58 patients (75.3%) had a histologic diagnosis of SCC at initial presentation. When comparing baseline characteristics according to the primary tumor location, patients with prostate primary (n=29, 37.7%) had a significantly higher proportion of patients with ED at initial presentation (75.9% vs. 18.8%, p < 0.001) and histologic transformation (55.2% vs. 6.3%, p < 0.001) compared to those with NP GU primary (n=48, 62.3%) (Table 2).
2. Clinical outcomesWith a median follow-up duration of 15.5 months (interquartile range, 7.1 to 34.4 months), the median PFS of patients with LD and ED was 47.5 months (95% CI, 9.7 to not available [NA]) and 6.7 months (95% CI, 4.9 to 10.8), respectively, and the 12-month PFS rates were 55.2% (95% CI, 42.0 to 72.4) and 26.7% (95% CI, 14.4 to 49.7), respectively (Fig. 1A). The median OS was 62.1 months (95% CI, 22.8 to NA) and 9.7 months (95% CI, 7.1 to 18.6), respectively, and the 24-month OS rates were 63.6% (95% CI, 49.9 to 81.0) and 15.6% (95% CI, 6.6 to 38.2), respectively (Fig. 1B). When comparing survival outcomes according to the primary tumor location (prostate vs. NP GU primary), there were no significant differences in terms of both PFS (log-rank, p=0.31) and OS (log-rank, p=0.69) among patients with LD, as well as those with ED (log-rank, p=0.43 and p=0.37, respectively) (Fig. 1C-F). We conducted multivariable Cox proportional hazards model analysis to evaluate independent prognostic variables associated with outcomes. Initial stage was the only variable associated with survival in terms of both PFS (adjusted hazard ratio [HR], 3.45 [95% CI, 1.89 to 6.25] for ED; adjusted p < 0.001) and OS (adjusted HR, 4.76 [95% CI, 2.63 to 9.09]; adjusted p < 0.001) (S1 Table).
When comparing outcomes according to platinum agent (carboplatin vs. cisplatin), there was no significant difference in survival outcomes regardless of stage (LD vs. ED) (S2 Fig.). Among patients with LD (n=46), 21 patients (45.7%) received EBRT with or without concurrent chemotherapy following induction etoposide plus platinum chemotherapy. The other 25 patients (54.3%) received surgery following neoadjuvant chemotherapy. There was no difference in OS according to the local treatment modalities (Fig. 2).
During the follow-up, 51 patients (66.2%) had a PFS event, including 27 patients with ED (87.1%) and 24 patients with LD (52.2%), and 26 of these patients (50.9%) received subsequent treatment (10 patients with ED and 16 patients with LD). Among patients with LD who received subsequent treatment (n=10), seven patients received CAV, consisting of cyclophosphamide, doxorubicin, and vincristine, one patient received docetaxel, one patient received nivolumab, and another patient received VIP, consisting of ifosfamide, etoposide, and cisplatin. Among patients with ED who received subsequent treatment (n=16), 10 patients received CAV, two patients received cisplatin plus irinotecan, one patient received TIP (consisting of paclitaxel, ifosfamide, and cisplatin), one patient received nivolumab, one patient had re-challenge of etoposide plus platinum, and another patient had paclitaxel plus cyclophosphamide. The median postprogression survival of patients who received subsequent treatment was 6.8 months (95% CI, 2.9 to NA) for patients with ED and 8.1 months (95% CI, 6.9 to 29.8) for patients with LD.
3. Tumor microenvironment immune cell profiling of NP GU SCCWe performed immune profiling of the tumor microenvironment using multiplex IHC analysis for 21 patients with NP GU SCC, including 14 patients (66.7%) with primary bladder SCC and seven patients (33.3%) with ureter SCC. The baseline characteristics of these patients are described in S3 Table, and representative slides of the multiplex IHC staining are shown in Fig. 3A and B. When comparing the immune cell population according to the initial stage (ED [n=4] vs. LD [n=17]), patients with LD had significantly higher lymphocyte density, including CD8+ T cell (p=0.009), CD8+CD103+tissue resident memory T cells (p=0.04), CD4+Foxp3– helper T cells (p=0.04), and CD20+ B cells (p=0.01) (Table 3).
We also analyzed the association of immune cell density and OS outcomes, and a higher density of PD-L1 expressing M1-like CD68+CD206– macrophages, PD-L1 expressing M2-like CD68+CD206+ macrophages, and total PD-L1 expressing cells were associated with poor outcomes (Fig. 3C). We dichotomized the patients according to PD-L1 expressing M1-like and M2-like macrophages density and total PD-L1 expressing cell density at the median and compared OS between the two groups. Patients with a density of PD-L1 expressing M1-like and M2-like macrophages, and total PD-L1 expressing cells higher than the median showed significantly worse OS outcomes compared to those lower than the median (Fig. 3D-F). Then, we performed multivariable analysis using Cox proportional hazards modeling with other clinical variables, and patients with a density of PD-L1 expressing M2-like CD68+CD206+ macrophages higher than the median had an independent association with poor OS outcomes, with an adjusted HR of 4.17 (95% CI, 1.25 to 14.29; adjusted p=0.02) (S4 Table).
DiscussionCurrently, there are only a limited number of studies reporting clinical features and outcomes of patients with primary GU SCC. Our study provides clinical outcomes of prostate or NP GU SCC with a relatively large sample size treated with a homogeneous regimen. More than half of the patients (59.7%) had an initial LD stage and were treated with etoposide plus platinum chemotherapy followed by EBRT or surgery with a median OS of 62.1 months. Previous studies showed the inferior survival of patients treated with surgery alone, and multimodality treatment, including neoadjuvant chemotherapy followed by local treatment, is widely accepted in treating patients with localized GU SCC [3,4,13,14]. Our study showed that EBRT and surgery may be done following induction chemotherapy based on the clinical situation, as there was no significant difference in outcomes between the two modalities. Overall, the 12-month PFS rate and the 24-month OS rate were 55.2% and 63.6%, respectively, in these patients with localized disease treated with a multimodal approach, implying a need for better therapeutic strategies.
All patients with ED were treated with first-line etoposide plus platinum doublet chemotherapy and showed poor outcomes with a median PFS and OS of 6.7 months and 9.7 months, respectively. There was no difference in survival outcomes according to the primary tumor location (prostate vs. NP GU). These discouraging outcomes are similar in previous reports with a median OS of 7-13 months among patients with bladder SCC ED, and 10.5 months among patients with prostate SCC ED treated with etoposide plus cisplatin and doxorubicin [4,5,15,16]. Among our total 77 patients, 51 patients (66.2%) progressed on initial treatment, and the median post-progression survival of patients who received subsequent chemotherapy was 7.6 months. Indeed, better treatment options should be investigated to improve the survival outcomes of patients with GU SCC. Immune checkpoint inhibitors may be a reasonable consideration as a treatment option given the fact that atezolizumab plus chemotherapy has shown significant benefit over chemotherapy alone in patients with SCLC ED [6,7]. However, only several case reports have shown the efficacy of checkpoint inhibitors for extrapulmonary SCC, and the immunologic features of extrapulmonary SCC, including GU SCC, has yet to be investigated [17,18].
From the tumor immune profiling analysis, patients with a higher density of PD-L1 expressing cells, especially PD-L1 expressing CD68+CD206+ M2-like macrophages, had significantly worse OS outcomes. Numerous preclinical data suggest that M2-polarized macrophages play crucial roles in tumor progression and therapeutic resistance, and M2-like macrophage phenotypes have shown an association with a poor prognosis in many types of cancers [19-21]. PD-L1 expression by the M2-like macrophages may elicit suppression of anti-tumor immunity, which may be associated with a poor prognosis [22]. On the other hand, as higher PD-L1 expression is well known to be a predictive biomarker of programmed death-1 (PD-1)/PD-L1 inhibitors in several cancers, and the addition of PD-1/PD-L1 inhibitors to the treatment of NP GU SCC may improve survival outcomes, especially for patients with high PD-L1 expressing CD68+CD206+ macrophages, which represents a subgroup with a poor prognosis on conventional chemotherapy [23]. However, considering the small sample size of the current study and that only multiplex IHC has been performed, further validation studies with deeper immune phenotyping are needed to evaluate the association between the macrophage PD-L1 expression, immune suppression, and prognosis of NP GU SCC.
When comparing the immune cell density of the tumor microenvironment according to the clinical stage (LD [n=17] vs. ED [n=4]), patients with LD had a significantly higher lymphocyte density, including CD8+ T cells and CD8+ T cells with a tissue resident memory T cell phenotype (CD103+). CD8+ T cells are the key players in anti-tumor immunity and are well known to be a predictor of an immune checkpoint inhibitor response. Tissue resident memory T cells are also associated with better CD8+ T cell responses [24,25]. These findings suggest that patients with localized disease may have a more favorable tumor microenvironment compared to those with advanced disease in terms of anti-tumor immunity and immune checkpoint inhibitor response. Similar findings were reported in triple-negative breast cancer: that tumor immunologic features change to a more tumor friendly and immunosuppressive environment with the progression and metastasis of the disease [26].
This study is a single center observational study, and only a small proportion of patients with NP GU SCC were included in the multiplex IHC analysis. However, our study provides valuable clinical data considering the rarity of GU SCC, and moreover, there are no previous studies evaluating the immune landscape of GU SCC. Our study underscores the poor prognosis of patients with GU SCC and the limited treatment options, along with the immunologic features of NP GU SCC associated with clinical outcomes.
In conclusion, patients with GU SCC showed a poor prognosis with a median survival of less than a year among patients with ED, and more than one-third of patients with LD died within two years despite multimodality treatment. Our multiplex IHC analysis described the immune cell phenotypes in the tumor microenvironment of NP GU SCC and showed an association with survival outcomes. These results may provide new insights for further investigations of biomarkers and immunotherapy for GU SCC.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement All procedures in studies involving human participants were performed in accordance with the ethical standards of the Institutional Review Board of Asan Medical Center and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards (IRB approval No. 2020-1346). The participants provided informed consents for the use of their clinical information and the analysis of their tumor samples, except for the deceased patients. Author Contributions Conceived and designed the analysis: Hyung J, Kim HD, Yoon S, Lee JL. Collected the data: Hyung J, Kim HD, Kim GH, Cho YM, Ryu YM, Kim SY, Park I, Yoon S, Lee JL. Contributed data or analysis tools: Hyung J, Kim HD, Kim GH, Cho YM, Ryu YM, Kim SY. Performed the analysis: Hyung J. Wrote the paper: Hyung J, Kim HD, Cho YM, Kim SY, Park I, Yoon S, Lee JL. Fig. 1.Kaplan-Meier estimates of survival outcomes according to stage (limited disease [LD] vs. extensive disease [ED]) and primary tumor location (prostate vs. non-prostate genitourinary GU). Progression-free survival (PFS) (A) and overall survival (OS) (B) in both LD and ED. PFS (C) and OS (D) according to the primary tumor location in patients with LD. PFS (E) and OS (F) according to the primary tumor location in patients with ED. CI, confidence interval. 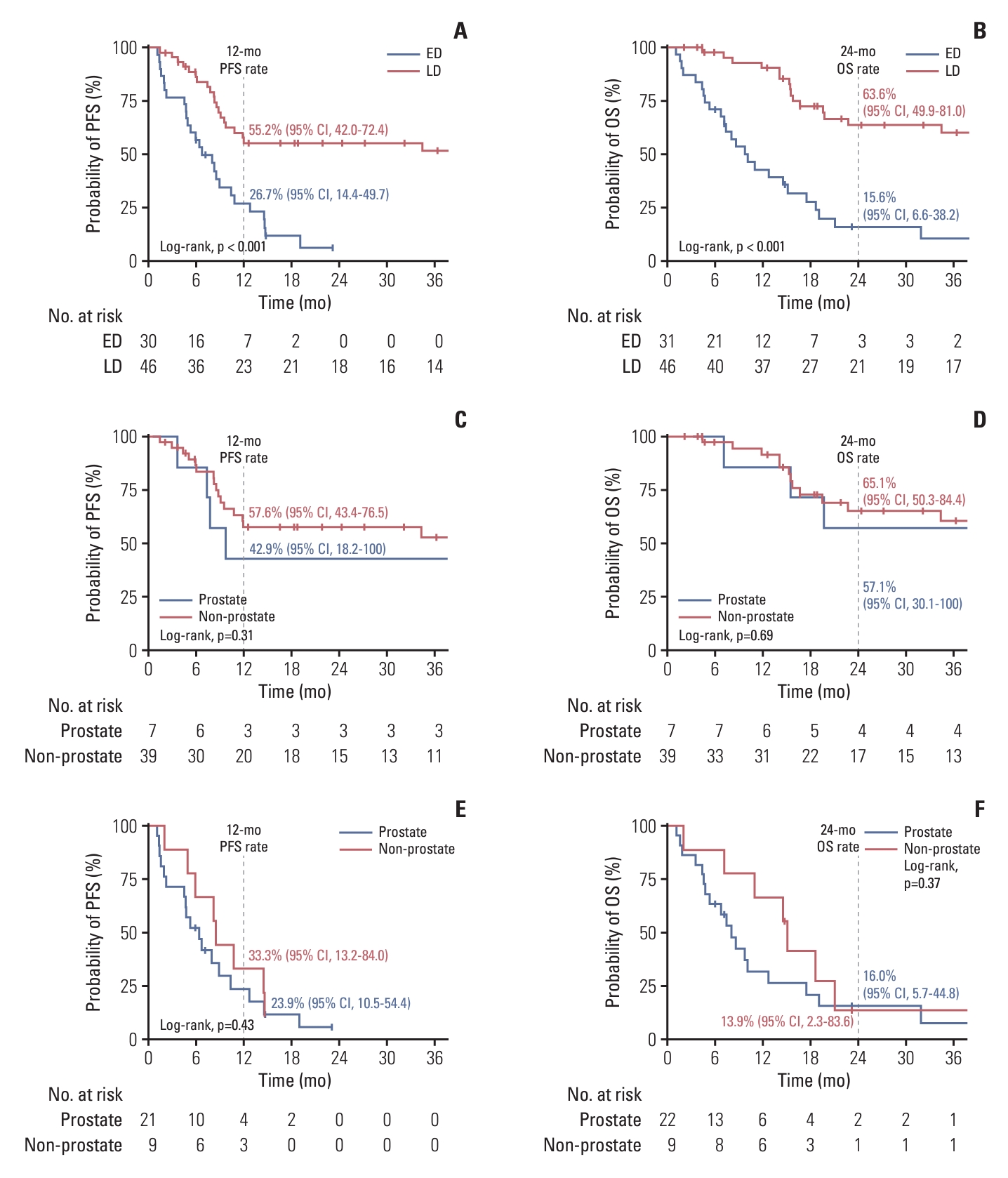
Fig. 2.Kaplan-Meier estimates of survival outcomes according to the local treatment modality (radiotherapy vs. surgery) following neoadjuvant etoposide plus platinum (EP) chemotherapy in patients with limited disease (n=46). (A) Progression-free survival (PFS). (B) Overall survival (OS). CCRT, concurrent chemoradiotherapy. 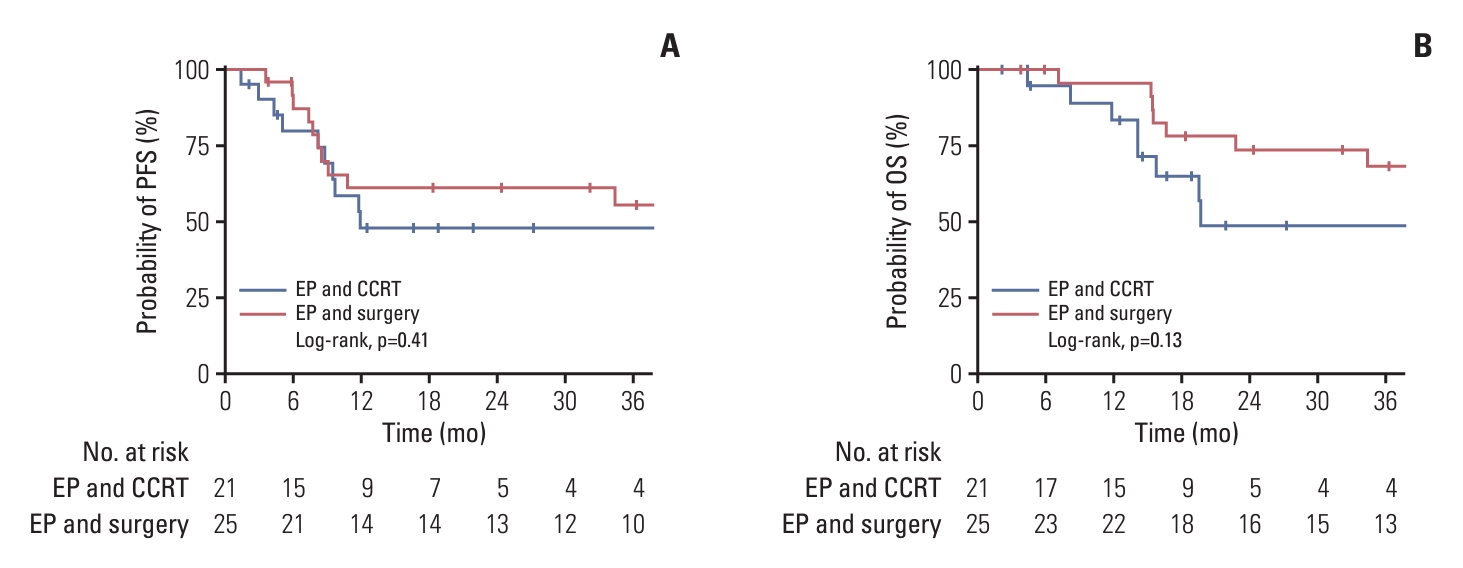
Fig. 3.(A, B) Representative slide images of multiplex immunohistochemistry. (C) Forest plot showing HRs of immune cell density and associations with OS analyzed by a Cox proportional hazards model. CI, confidence interval; DC, dendritic cell; HR, hazard ratio; OS, overall survival; PD-L1, programmed death-ligand 1. Kaplan-Meier estimates of OS according to immune cell density dichotomized at the median (D-F). (D) Programmed death-ligand 1 (PD-L1) expressing CD68+CD206+ M2-like macrophages. (E) PD-L1 expressing CD68+CD206– M1-like macrophages. (F) PD-L1 expressing cells. 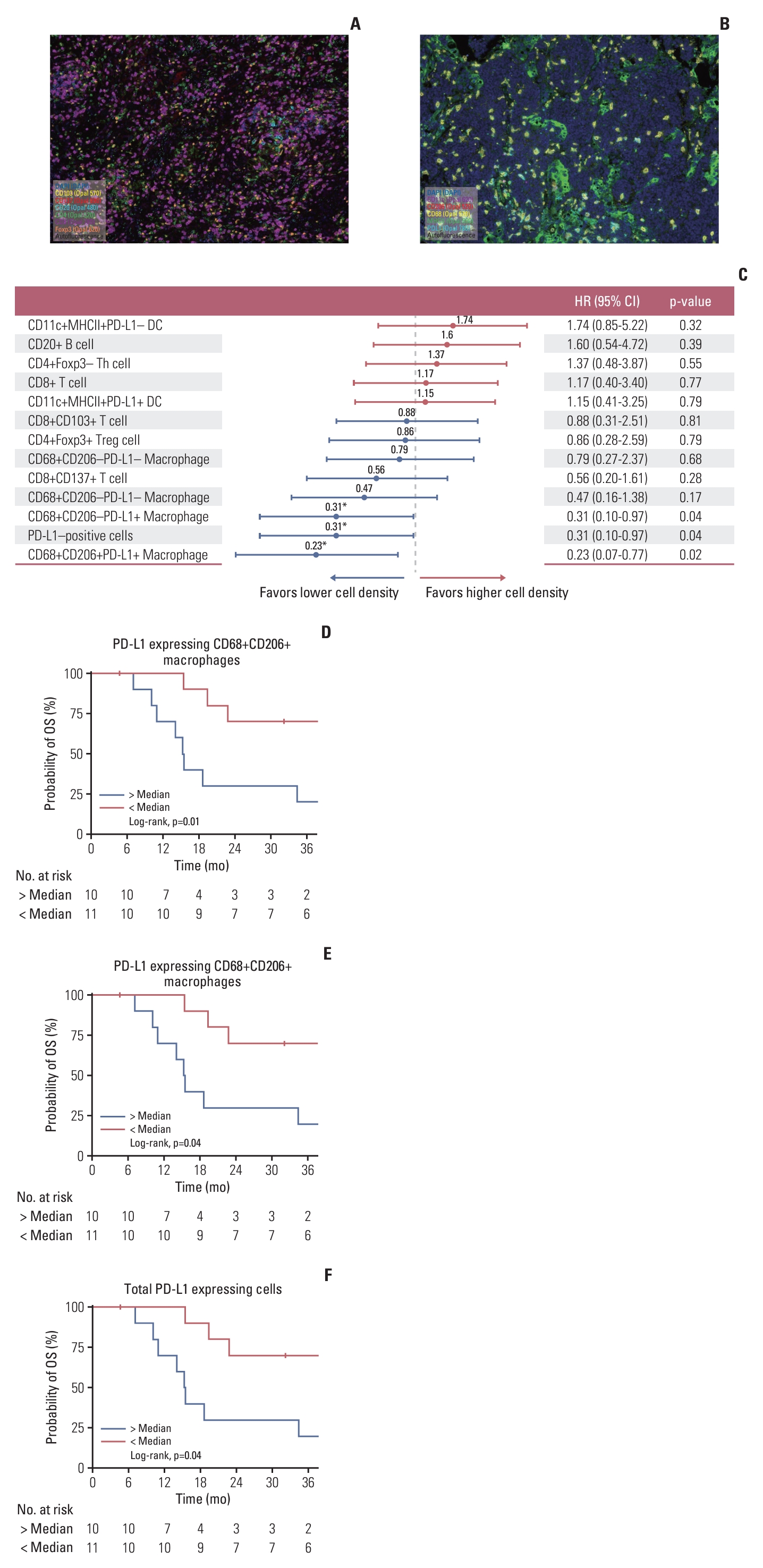
Table 1.Baseline characteristics of small cell carcinoma of the genitourinary system
Table 2.Comparison of clinical characteristics according to the primary tumor location Table 3.Comparison of immune cell density (cells/mm2) according to clinical stage References1. Brennan SM, Gregory DL, Stillie A, Herschtal A, Mac Manus M, Ball DL. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010;116:888–95.
2. Gupta K, Gupta S. Neuroendocrine differentiation in prostate cancer: key epigenetic players. Transl Cancer Res. 2017;6:S104–8.
3. Thota S, Kistangari G, Daw H, Spiro T. A clinical review of small-cell carcinoma of the urinary bladder. Clin Genitourin Cancer. 2013;11:73–7.
4. Lynch SP, Shen Y, Kamat A, Grossman HB, Shah JB, Millikan RE, et al. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. Eur Urol. 2013;64:307–13.
5. Ismaili N, Heudel PE, Elkarak F, Kaikani W, Bajard A, Ismaili M, et al. Outcome of recurrent and metastatic small cell carcinoma of the bladder. BMC Urol. 2009;9:4.
6. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9.
7. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22:51–65.
8. Cicin I, Karagol H, Uzunoglu S, Uygun K, Usta U, Kocak Z, et al. Extrapulmonary small-cell carcinoma compared with small-cell lung carcinoma: a retrospective single-center study. Cancer. 2007;110:1068–76.
9. Shen P, Jing Y, Zhang R, Cai MC, Ma P, Chen H, et al. Comprehensive genomic profiling of neuroendocrine bladder cancer pinpoints molecular origin and potential therapeutics. Oncogene. 2018;37:3039–44.
10. Chang MT, Penson A, Desai NB, Socci ND, Shen R, Seshan VE, et al. Small-cell carcinomas of the bladder and lung are characterized by a convergent but distinct pathogenesis. Clin Cancer Res. 2018;24:1965–73.
11. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–6.
12. Koay EJ, Teh BS, Paulino AC, Butler EB. A Surveillance, Epidemiology, and End Results analysis of small cell carcinoma of the bladder: epidemiology, prognostic variables, and treatment trends. Cancer. 2011;117:5325–33.
13. Siefker-Radtke AO, Dinney CP, Abrahams NA, Moran C, Shen Y, Pisters LL, et al. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J Urol. 2004;172:481–4.
14. Wang Y, Li Q, Wang J, Tong M, Xing H, Xue Y, et al. Small cell carcinoma of the bladder: the characteristics of molecular alterations, treatment, and follow-up. Med Oncol. 2019;36:98.
15. Yamada Y, Beltran H. Clinical and biological features of neuroendocrine prostate cancer. Curr Oncol Rep. 2021;23:15.
16. Papandreou CN, Daliani DD, Thall PF, Tu SM, Wang X, Reyes A, et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized smallcell carcinoma of the prostate. J Clin Oncol. 2002;20:3072–80.
17. Husnain M, Park W, Ramos JC, Johnson TE, Chan J, Dasari A, et al. Complete response to ipilimumab and nivolumab therapy in a patient with extensive extrapulmonary highgrade small cell carcinoma of the pancreas and HIV infection. J Immunother Cancer. 2018;6:66.
18. Ugwu JK, Nwanyanwu C, Shelke AR. Dramatic response of a metastatic primary small-cell carcinoma of the pancreas to a trial of immunotherapy with nivolumab: a case report. Case Rep Oncol. 2017;10:720–5.
19. Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22:6995.
20. Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400.
21. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95.
22. Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. 2021;6:127.
23. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–50.
24. Lee JS, Ruppin E. Multiomics prediction of response rates to therapies to inhibit programmed cell death 1 and programmed cell death 1 ligand 1. JAMA Oncol. 2019;5:1614–8.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||