AbstractPurposeThis study aimed to investigate the incidence and risk factors for secondary malignant neoplasms (SMN) in pediatric solid tumors, focusing on the effects of tandem high-dose chemotherapy (HDCT).
Materials and MethodsPatients (aged < 19 years) diagnosed with or treated for pediatric solid tumors between 1994 and 2014 were retrospectively analyzed. The cumulative incidence of SMN was estimated using competing risk methods by considering death as a competing risk.
ResultsA total of 1,435 patients (413 with brain tumors and 1,022 with extracranial solid tumors) were enrolled. Seventy-one patients developed 74 SMNs, with a 10-year and 20-year cumulative incidence of 2.680±0.002% and 10.193±0.024%, respectively. The types of SMN included carcinoma in 28 (37.8%), sarcoma in 24 (32.4%), and hematologic malignancy in 15 (20.3%) cases. Osteosarcoma and thyroid carcinoma were the most frequently diagnosed tumors. Multivariate analysis showed that radiotherapy (RT) > 2, 340 cGy, and tandem HDCT were significant risk factors for SMN development. The SMN types varied according to the primary tumor type; carcinoma was the most frequent SMN in brain tumors and neuroblastoma, whereas hematologic malignancy and sarcomas developed more frequently in patients with sarcoma and retinoblastoma, respectively.
ConclusionThe cumulative incidence of SMN in pediatric patients with solid tumors was considerably high, especially in patients who underwent tandem HDCT or in those who received RT > 2,340 cGy. Therefore, the treatment intensity should be optimized based on individual risk assessment and the long-term follow-up of pediatric cancer survivors.
IntroductionThe survival rates of pediatric patients with cancer have improved significantly over the decades, resulting in a 5-year survival rate of > 80% [1]. This improvement has been attributed to the development of various treatment protocols, supportive care, and modern multimodal treatment approaches. However, this improvement was accompanied by an increase in various late complications among long-term survivors. The development of secondary malignant neoplasms (SMN) is one of the most serious complications for survivors. The Childhood Cancer Survivor Study (CCSS) showed that pediatric cancer survivors had an excess mortality rate long after diagnosis, and the death rate due to SMN was the highest after death attributed to recurrent disease [2].
Autologous hematopoietic cell transplantation and high-dose chemotherapy (HDCT) are widely used to improve the survival rate of pediatric patients with high-risk or relapsed solid tumors [3-5]. In our institution, we implemented tandem HDCT to improve the treatment outcomes of patients with high-risk solid tumors [6-9] and minimize radiation exposure in patients with brain tumors [7,10], which has shown some success, especially in improving survival. However, because HDCT regimens for solid tumors usually include high-dose alkylating agents and, in some cases, total body irradiation (TBI), HDCT could be a risk factor for SMN development.
In this study, we evaluate the incidence and risk factors of SMN in pediatric patients with solid tumors, focusing on the effects of tandem HDCT.
Materials and Methods1. Patients and data collectionPediatric patients diagnosed with or treated for solid tumors at the Samsung Medical Center between November 1994 and December 2014, who were under 19 years of age at initial diagnosis, were included in this study. An SMN was defined as a histologically distinct tumor that was diagnosed at least 6 months after the diagnosis of a primary tumor [11,12]. Patients with a follow-up duration of less than 6 months and those who visited our hospital after an SMN diagnosis were excluded. Clinical data regarding the characteristics of the primary tumors, treatment modalities, SMN characteristics, and treatment outcomes were retrospectively collected. Primary tumors are primarily divided into brain and extracranial solid tumors. Brain tumors are subdivided into embryonal tumors, intracranial germ cell tumors, low-grade gliomas, high-grade gliomas, ependymomas, and other rare tumors. Extracranial solid tumors include neuroblastomas, retinoblastomas, sarcomas, Wilms tumors, germ cell tumors, and hepatoblastomas/hepatocellular carcinomas. Treatment modalities were categorized into surgery, chemotherapy, radiotherapy (RT), and HDCT. The use of chemotherapeutic agents such as cyclophosphamide, ifosfamide, cisplatin, etoposide, and doxorubicin and data on the regimen in patients who underwent HDCT were investigated. When patients were tested for cancer-predisposing genes, the results were collected regardless of the testing method, which included candidate gene testing or panel sequencing. SMN were primarily categorized as carcinoma, hematologic malignancy, and sarcoma. The time to SMN development and outcomes after SMN development were also recorded.
2. Statistical analysisClinical characteristics were compared between the two groups using the Pearson chi-square test or Fisher’s exact test for categorical variables and the Kruskal-Wallis ranksum test for continuous variables. The cumulative incidence of SMN was estimated using competing risk methods by considering death as a competing risk [13]. The cumulative incidence of SMN was compared using Gray’s test. A cause-specific Cox regression model was used to evaluate the effects of clinical covariates on the cumulative incidence of SMN. Maximally selected log-rank statistics were used to obtain the optimal RT cutoff dose to separate patients in terms of SMN incidence [14]. All statistical analyses were performed using R ver. 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/), and p-values < 0.05 were considered statistically significant.
Results1. Patients’ characteristicsA total of 1,435 pediatric patients with solid tumors were enrolled in this study. Seventy-one patients (4.9%) developed SMN. The number and percentage of patients who developed SMN according to their characteristics are summarized in Table 1. The follow-up duration was longer in patients with SMN (median, 15.2 vs. 9.1 years; p < 0.001). In this study population, 354 patients died of primary or secondary tumor progression or treatment-related mortality, resulting in a 10-year overall rate of 75.1%. The median follow-up duration was 9.5 years (range, 0.5 to 26.3 years), and the median age at the last follow-up was 15.5 years (range, 0.7 to 41.7 years).
2. Incidence and characteristics of SMNSeventy-one patients developed 74 SMNs, including three patients who developed two SMNs sequentially. The 10- and 20-year cumulative incidence of SMN was 2.680±0.002% and 10.193±0.024%, respectively. The median time to the development of SMN was 11.6 years (range, 1.3 to 25.3 years). Carcinoma accounted for 28 cases (37.8%), followed by sarcoma in 24 cases (32.4%), and hematologic malignancy in 15 cases (20.3%). Time to SMN was the shortest in hematologic malignancy, with a median of 3.0 years (range, 1.3 to 18.9 years) compared to those of carcinoma (median, 12.7 years; range, 5.1 to 25.3 years) or sarcoma (median, 12.2 years; range, 3.2 to 19.0 years) (p < 0.001) (S1 Table). SMN-related deaths occurred in 18 patients, resulting in 0.8% of 10-year and 3.6% of 20-year cumulative incidences. Five-year overall survival after SMN development was 64.4±7.3%. Survival after SMN diagnosis was highest in the carcinoma group, with 92.0±5.5% of the 5-year overall survival rate (p=0.026) (Fig. 1).
Among the 74 cases of SMN, osteosarcoma and thyroid carcinoma were the most frequently diagnosed in 16 and 12 patients, respectively. Secondary osteosarcomas developed in nine patients with bilateral retinoblastoma, three with high-risk neuroblastoma, two with adrenocortical carcinoma, and one with medulloblastoma and Ewing sarcoma. Secondary osteosarcoma following Ewing sarcoma developed within the previous radiation field, and two patients with adrenocortical carcinoma were confirmed to have Li-Fraumeni syndrome with a germline TP53 pathogenic variant. Among the 12 patients with thyroid carcinomas, 10 had papillary thyroid carcinomas, and two cases had follicular thyroid carcinomas. The primary tumors were neuroblastoma (six patients), brain tumors (two patients), and others (four patients). Among them, six patients received RT in the neck area.
3. Risk factors of SMNClinical characteristics, including treatment modalities and diagnosis, were analyzed as risk factors for SMN (Fig. 2). There was no difference in the cumulative incidence of SMNs when the patients were divided into two groups based on whether they received RT. To consider the effect of the RT dose, a maximally selected log-rank test was performed, and the cutoff value that resulted in a difference in SMN incidence was 2,340 cGy. The SMN incidence was higher in patients who received RT exceeding 2,340 cGy (p=0.016), whereas patients who received RT ≥ 2,340 cGy showed no difference from those who did not receive RT. The 20-year cumulative incidences of SMN according to HDCT were 5.930±0.015% in patients without HDCT, 8.567±0.151% in patients who underwent single HDCT, and 18.453±0.186% in patients with tandem HDCT. HDCT was a risk factor for SMN (p=0.004). No significant difference in SMN incidence was observed between patients who received a single HDCT and those who did not receive HDCT; the incidence of SMN was significantly increased in patients who received tandem HDCT (p=0.001). Patients with cancer predisposition syndrome (CPS) had a higher risk of developing SMN (p=0.011). Among chemotherapeutic agents, only cyclophosphamide and ifosfamide were risk factors for SMN development (p=0.013 and p=0.001, respectively). In a subgroup analysis of patients who underwent HDCT, there was no difference in the incidence of SMN according to the specific conditioning drug or modality used. The incidence of SMN was higher in patients with extracranial solid tumors than in those with brain tumors (p=0.033) (Fig. 3).
In the multivariate analysis using treatment modalities and diagnosis as covariates, extracranial solid tumors, RT > 2,340 cGy, and tandem HDCT were significant factors for developing SMNs (Table 2). The presence of CPS was excluded from the multivariate analysis because of the high number of missing values.
We conducted additional analyses to investigate whether the characteristics of SMN, such as SMN type, location, and timing, differed depending on the presence of risk factors, such as RT and HDCT. There were no differences in the timing or site of occurrence of SMN based on RT or HDCT. However, SMN types showed significant differences based on RT dose (p=0.045), and although there was no statistically significant difference in SMN types based on HDCT, there was a trend of increase in sarcomas with the number of HDCT (p=0.605) (S2 Fig.).
Among the 48 SMN cases previously treated with RT, excluding the 11 cases of hematologic malignancy, 24 of the 37 SMN cases occurred within the previous radiation field, and 13 occurred outside the field. The specific numbers according to SMN type are detailed in S2 Fig.
4. SMN according to the primary tumor typeThe cumulative incidence of SMN according to tumor type is illustrated in Fig. 3. Among the brain tumors, embryonal tumors showed the highest cumulative incidence, although the difference was not statistically significant. In extracranial solid tumors, there was a statistically significant difference in the cumulative incidence of SMN according to the diagnosis (p=0.028). The SMN type differed according to the primary tumor type (p < 0.001) (Fig. 4). Carcinoma is the most frequent SMN among brain tumors and neuroblastomas, whereas hematologic malignancies and sarcomas frequently develop into sarcomas and retinoblastomas, respectively. Detailed information on the SMN types according to primary tumor type is provided in S3 Table.
Among 413 patients with brain tumors, 14 SMNs occurred in 13 patients (3.1%), and the primary diagnoses were embryonal tumors in nine patients, intracranial germ cell tumors in three patients, and low-grade glioma in one patient. The 10- and 20-year cumulative incidences of SMN were 1.745±0.005% and 5.138±0.027%, respectively. There were 79 patients who did not receive RT and 17 patients who received RT ≤ 2,340 cGy, and none of them developed SMN. All patients who developed SMN received RT > 2,340 cGy. Among them, 26 patients underwent HDCT (24 tandem HDCT and two single HDCT) to omit RT or reduce the RT dose, and no SMN occurred in these patients. From the perspective of the RT field, the incidence of SMN was significantly higher in patients who underwent craniospinal irradiation (p=0.037). HDCT showed no statistically significant difference in the incidence of SMN development in the brain tumor subgroup (S4 Fig.).
In 403 patients with neuroblastoma, 26 SMNs developed in 25 patients (6.2%), resulting in 10- and 20-year cumulative incidences of 2.577±0.007% and 17.133±0.195%, respectively. Among these patients, 20 underwent HDCT; a TBI-based HDCT regimen was used in 12 patients, and a non-TBI–based regimen was used in eight patients. RT, especially RT > 2,340 cGy, was a significant factor in the development of SMN in neuroblastoma (S5 Fig.). In the multivariate analysis confined to neuroblastoma using RT and HDCT as covariates, RT > 2,340 cGy showed statistical significance (p=0.006), whereas no significant difference was observed with HDCT (p=0.764). HDCT regimens, including TBI and metaiodobenzylguanidine, did not show statistical significance (p=0.763) (S5 Fig.).
In 222 patients with sarcoma, 15 SMNs developed in 14 patients (6.3%), including eight hematologic malignancies, but there was no significant difference in the cumulative incidence of SMNs according to disease category and RT history. Among the 134 patients with retinoblastoma, SMN occurred in 12, 11 of whom had bilateral retinoblastomas. One of the 96 patients with unilateral retinoblastoma developed thyroid carcinoma as an SMN, and 11 of the 38 patients with bilateral retinoblastoma developed osteosarcoma (nine patients) and non-rhabdomyosarcoma soft tissue sarcoma (two patients). Among patients with bilateral retinoblastoma, those who underwent HDCT showed a higher incidence of SMNs (p=0.031) (S6 Fig.). Among the 82 patients with Wilms tumors, no SMN occurred, including 46 patients who received RT.
DiscussionIn this study, the cumulative incidence of SMN in pediatric patients with solid tumors was considerably high, with a 10- and 20-year cumulative incidence of 2.680±0.002% and 10.193±0.024%, respectively. This incidence is higher than that reported in previous studies. CCSS has a 20-year cumulative incidence of 3.2% [15]. A follow-up study by the same group showed a 30-year cumulative incidence of 20.5%, although the incidence of SMN was 7.9%, excluding nonmelanoma skin cancer [16]. A study from Japan also showed 10- and 20-year cumulative incidences of 1.1% and 2.6%, respectively [17], and a nationwide population-based study using the National Cancer Registry in Korea showed 10- and 15-year cumulative incidences of 1.2% and 2.0%, respectively [11]. This result is partly attributed to the differences in the study population. Our study included only patients with solid tumors, whereas other studies included all pediatric cancers, including hematologic malignancies. In a study by a Japanese group [17], the cumulative incidence is the lowest in hematologic malignancy, indicating that it could be somewhat higher if confined to solid tumors. In contrast, CCSS indicated that the highest incidence of SMN was in Hodgkin’s disease [15,16]. Another possible explanation for the high incidence of SMN in our study could be that a high proportion of patients received HDCT (33.4%), and 80.4% of them received tandem HDCT. Considering that HDCT was confirmed as a risk factor for the development of SMN, this could be a possible explanation.
The development of SMNs after HDCT has been investigated in several studies. In a previous study by Baker et al. [18] involving 3,372 patients who underwent hematopoietic stem cell transplantation (45% < 20 years of age and 35% receiving autologous stem cell transplantation), the 20-year cumulative incidence of SMN was 6.9%. Another study on children who underwent HDCT showed a 2.6% 10-year cumulative incidence of SMN [19]. However, in these two studies, the incidence of SMNs was not compared with that in patients without stem cell transplantation. In a recent study on neuroblastoma, high-risk patients showed a greater cumulative incidence of SMN than low- or intermediate-risk patients, suggesting that an intensive multimodal treatment strategy, such as HDCT, could be associated with SMN risk [20]. Regarding the HDCT regimen, the effects of TBI exposure on the incidence of SMN are controversial [18,19,21,22]. In our study, HDCT was found to be a risk factor for SMN development, and further analysis using the number of HDCT scans revealed that tandem HDCT was a stronger risk factor. Autologous hematopoietic cell transplantation and HDCT are well-established treatment options for pediatric patients with high-risk or relapsed solid tumors, including neuroblastoma [3-5] and brain tumors [23]. A recent phase III Children’s Oncology Group trial showed increased event-free survival using tandem HDCT [5]. Our center has actively performed tandem HDCT to improve the treatment outcomes for high-risk solid tumors [6-9], which resulted in increased survival. However, based on the results of this study, tandem HDCT should be implemented cautiously based on individual risk assessments.
RT is associated with an increased risk of SMN [16,24,25]. The CCSS showed that ionizing radiation exposure was associated with a 2.7 relative risk of developing any SMN and was the strongest independent risk factor for SMN development [16]. Several studies have investigated the effects of different RT doses. In a study of survivors of Ewing’s sarcoma, Kuttesch et al. [26] showed a dose-dependent increase in the risk of secondary sarcoma development, especially at doses > 6,000 cGy. Another study on patients with Hodgkin disease showed that increasing the radiation dose was associated with an increased risk of SMN [27]. In the present study, RT > 2,340 cGy was a risk factor for SMN development, whereas no difference was observed in the development of SMN in patients who received RT ≤ 2,340 cGy compared to those who did not receive RT. Our center has adopted a strategy to reduce the RT dose to decrease late sequelae in pediatric cancer survivors, especially those with brain tumors, including intracranial germ cell tumors and medulloblastomas [7,28,29]. As RT is an important modality in the treatment of pediatric cancer, its use is inevitable; however, reducing the radiation dose whenever possible is crucial.
CPS was a risk factor for SMN development in the present study, which is consistent with the findings of previous studies [30,31]. One of the interesting findings in our study was that patients with bilateral retinoblastoma who underwent HDCT showed a higher incidence than those with bilateral retinoblastoma without HDCT. Considering that bilateral retinoblastoma has a genetic predisposition, the incidence might vary according to the intensity of treatment in patients with a genetic risk. Therefore, the treatment intensity should be optimized, especially for patients with genetic risks.
In the present study, osteosarcomas were the most common SMNs. Secondary osteosarcoma developed in nine patients with bilateral retinoblastoma and two patients with Li-Fraumeni syndrome. Among the nine retinoblastoma patients, eight patients underwent HDCT (7 single and 1 tandem), and another four patients with neuroblastoma or medulloblastoma developed secondary osteosarcoma after HDCT. Considering the fact that HDCT has been identified as a risk factor for SMN development in bilateral retinoblastoma, additional research will be needed to explore the impact of HDCT on the development of secondary osteosarcoma.
This study has some limitations because of its retrospective nature. Patients with SMN may have been more cooperative with follow-up clinic visits, whereas those without SMN may have been censored earlier. In our cohort, a longer follow-up period was observed in patients with SMN, which could affect the later estimates of the cumulative incidence of SMN. Another limitation was the identification of the CPS. Because the indications, methods, and timing for the testing of cancer-predisposing genes vary depending on the period and the patient’s situation, CPS results need to be interpreted cautiously.
Despite these limitations, our study is noteworthy because it was conducted on a unique cohort of patients who underwent HDCT, especially tandem HDCT. The cumulative incidence of SMN in pediatric patients with solid tumors was considerably high, especially in those who underwent tandem HDCT or received RT > 2,340 cGy. Therefore, the treatment intensity should be optimized according to individual risk assessments, and long-term follow-up of pediatric cancer survivors is required.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No. SMC 2020-03-003). The requirement for informed consent was waived by the board due to the retrospective nature of the study. Author Contributions Conceived and designed the analysis: Lim H, Im M, Cho HW, Ju HY, Kim JW, Lim DH, Sung KW, Lee JW. Collected the data: Lim H, Im M, Seo ES, Cho HW, Ju HY, Yoo KH, Cho SY, Kim JW, Lim DH, Sung KW, Lee JW. Contributed data or analysis tools: Lim H, Im M, Seo ES, Cho HW, Ju HY, Sung KW, Lee JW. Performed the analysis: Lim H, Im M, Seo ES, Cho HW, Ju HY, Yoo KH, Cho SY, Kim JW, Lim DH, Lee JW. Wrote the paper: Lim H, Im M, Seo ES, Cho HW, Ju HY, Yoo KH, Cho SY, Kim JW, Lim DH, Sung KW, Lee JW. Visualized the data: Lim H, Im M, Ju HY, Lee JW. AcknowledgmentsThis work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (2020R1A2C1012723) and National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1720270).
Fig. 1. Overall survival after secondary malignant neoplasm (SMN) development. Survival after an SMN diagnosis differed according to SMN type. Overall survival after SMN was highest in the carcinoma group. 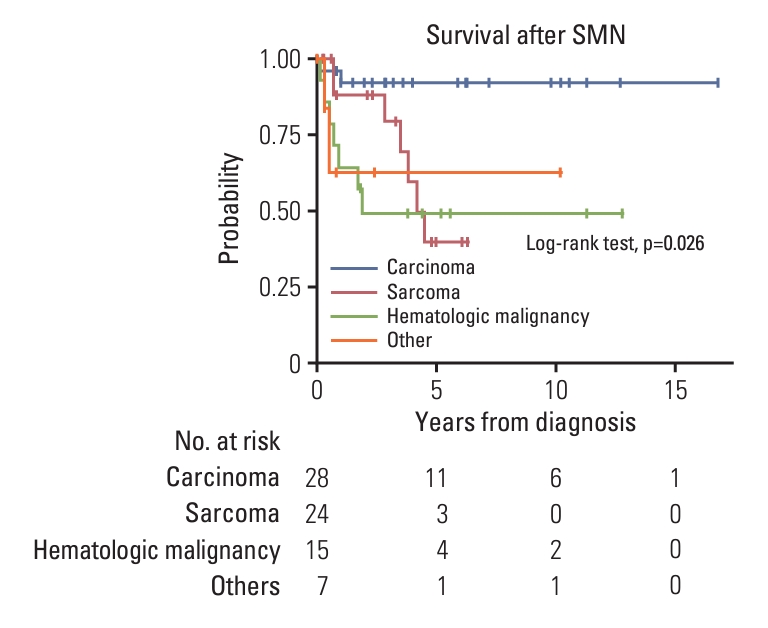
Fig. 2.Risk factors of secondary malignant neoplasm (SMN) development. Clinical characteristics, including treatment modalities and diagnosis, were analyzed as risk factors of SMN. The SMN incidence was higher in patients who received radiotherapy (RT) exceeding 2,340 cGy or tandem high-dose chemotherapy (HDCT), or in those with confirmed cancer predisposition syndromes. Among chemotherapeutic (CTx) agents, the use of cyclophosphamide and ifosfamide were risk factors for SMN development. 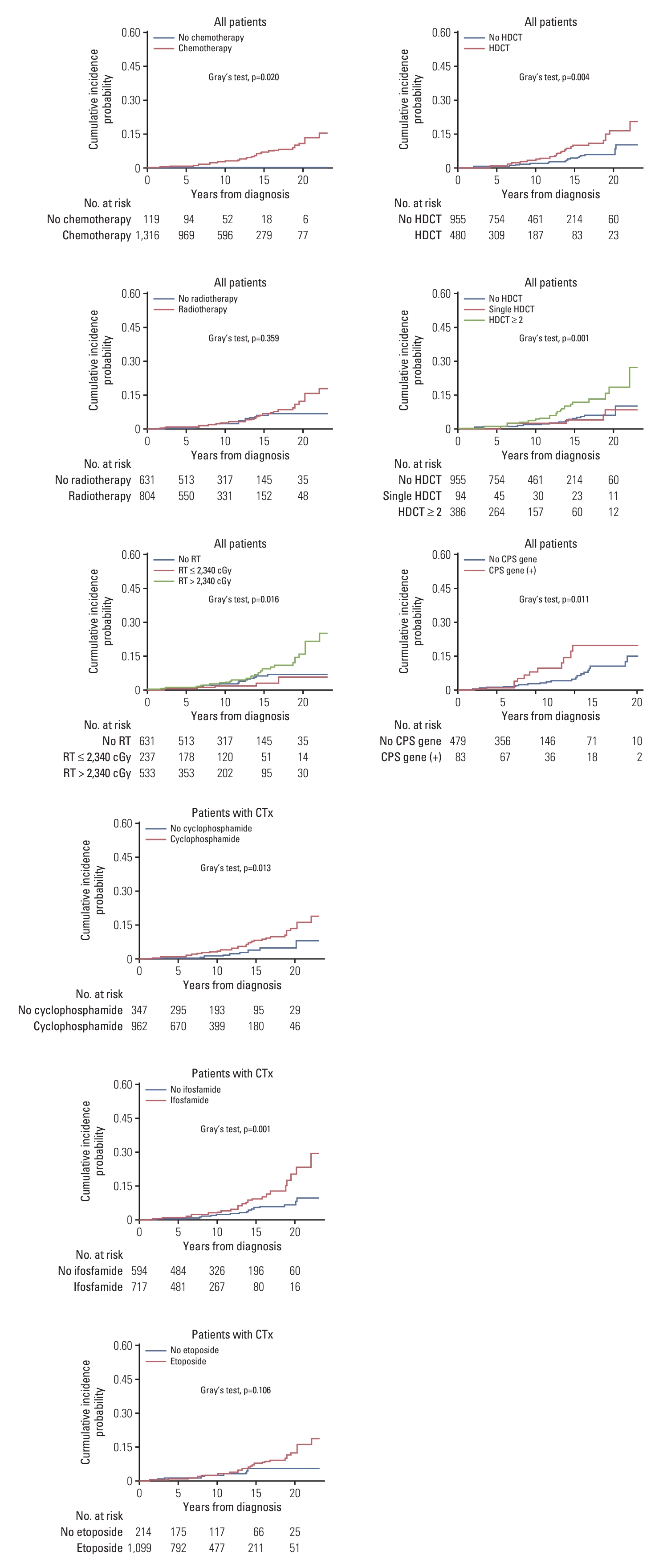
Fig. 3.Cumulative incidences of secondary malignant neoplasm (SMN) according to tumor type. The incidence of SMN was higher in patients with extracranial solid tumors than in those with brain tumors, and the incidences are illustrated according to tumor type. IC-GCT, intracranial germ cell tumor. 
Fig. 4.Secondary malignant neoplasm (SMN) type according to primary diagnosis. The SMN types were different according to primary tumor type. Carcinoma was the most frequent SMN in brain tumors and neuroblastomas, whereas hematologic malignancy (HM) and sarcoma frequently developed in patients with sarcoma and retinoblastoma, respectively. 
Table 1.Patient characteristics Table 2.Multivariate analysis of risk factors for SMN References1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
2. Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–79.
3. Kletzel M, Katzenstein HM, Haut PR, Yu AL, Morgan E, Reynolds M, et al. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J Clin Oncol. 2002;20:2284–92.
4. Matthay KK. Intensification of therapy using hematopoietic stem-cell support for high-risk neuroblastoma. Pediatr Transplant. 1999;3 Suppl 1:72–7.
5. Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: a randomized clinical trial. JAMA. 2019;322:746–55.
6. Lee JW, Lee S, Cho HW, Ma Y, Yoo KH, Sung KW, et al. Incorporation of high-dose (131)I-metaiodobenzylguanidine treatment into tandem high-dose chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma: results of the SMC NB-2009 study. J Hematol Oncol. 2017;10:108.
7. Sung KW, Lim DH, Son MH, Lee SH, Yoo KH, Koo HH, et al. Reduced-dose craniospinal radiotherapy followed by tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk medulloblastoma. Neuro Oncol. 2013;15:352–9.
8. Sung KW, Lim DH, Yi ES, Choi YB, Lee JW, Yoo KH, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation for atypical teratoid/rhabdoid tumor. Cancer Res Treat. 2016;48:1408–19.
9. Sung KW, Son MH, Lee SH, Yoo KH, Koo HH, Kim JY, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk neuroblastoma: results of SMC NB-2004 study. Bone Marrow Transplant. 2013;48:68–73.
10. Ma Y, Lim DH, Cho H, Lee JW, Sung KW, Yoo KH, et al. Tandem high-dose chemotherapy without craniospinal irradiation in treatment of non-metastatic malignant brain tumors in very young children. J Korean Med Sci. 2020;35:e405
11. Ju HY, Moon EK, Lim J, Park BK, Shin HY, Won YJ, et al. Second malignant neoplasms after childhood cancer: A nationwide population-based study in Korea. PLoS One. 2018;13:e0207243
12. Hammal DM, Bell CL, Craft AW, Parker L. Second primary tumors in children and young adults in the North of England (1968-99). Pediatr Blood Cancer. 2005;45:155–61.
13. Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13:559–65.
14. Laska E, Meisner M, Wanderling J. A maximally selected test of symmetry about zero. Stat Med. 2012;31:3178–91.
15. Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–29.
16. Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–95.
17. Ishida Y, Qiu D, Maeda M, Fujimoto J, Kigasawa H, Kobayashi R, et al. Secondary cancers after a childhood cancer diagnosis: a nationwide hospital-based retrospective cohort study in Japan. Int J Clin Oncol. 2016;21:506–16.
18. Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–8.
19. Danner-Koptik KE, Majhail NS, Brazauskas R, Wang Z, Buchbinder D, Cahn JY, et al. Second malignancies after autologous hematopoietic cell transplantation in children. Bone Marrow Transplant. 2013;48:363–8.
20. Applebaum MA, Vaksman Z, Lee SM, Hungate EA, Henderson TO, London WB, et al. Neuroblastoma survivors are at increased risk for second malignancies: a report from the International Neuroblastoma Risk Group Project. Eur J Cancer. 2017;72:177–85.
21. Forrest DL, Nevill TJ, Naiman SC, Le A, Brockington DA, Barnett MJ, et al. Second malignancy following high-dose therapy and autologous stem cell transplantation: incidence and risk factor analysis. Bone Marrow Transplant. 2003;32:915–23.
22. Vaxman I, Ram R, Gafter-Gvili A, Vidal L, Yeshurun M, Lahav M, et al. Secondary malignancies following high dose therapy and autologous hematopoietic cell transplantation-systematic review and meta-analysis. Bone Marrow Transplant. 2015;50:706–14.
23. Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–20.
24. Goldsby R, Burke C, Nagarajan R, Zhou T, Chen Z, Marina N, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer. 2008;113:2597–604.
25. Kaatsch P, Reinisch I, Spix C, Berthold F, Janka-Schaub G, Mergenthaler A, et al. Case-control study on the therapy of childhood cancer and the occurrence of second malignant neoplasms in Germany. Cancer Causes Control. 2009;20:965–80.
26. Kuttesch JF Jr, Wexler LH, Marcus RB, Fairclough D, Weaver-McClure L, White M, et al. Second malignancies after Ewing’s sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14:2818–25.
27. Constine LS, Tarbell N, Hudson MM, Schwartz C, Fisher SG, Muhs AG, et al. Subsequent malignancies in children treated for Hodgkin’s disease: associations with gender and radiation dose. Int J Radiat Oncol Biol Phys. 2008;72:24–33.
28. Lee JW, Lim DH, Sung KW, Cho HW, Ju HY, Yoo KH, et al. Induction chemotherapy reduces radiation therapy dose and volume in the treatment of intracranial germinoma: results of the SMC-G13 trial. Int J Radiat Oncol Biol Phys. 2020;108:649–56.
29. Lim DH, Yoo KH, Lee NH, Lee SH, Sung KW, Koo HH, et al. Intensive chemotherapy followed by reduced-dose radiotherapy for biopsy-proven CNS germinoma with elevated beta-human chorionic gonadotropin. J Neurooncol. 2014;117:279–85.
|
|
||||||||||||||||||||||||||||||||||||||||||