AbstractPurposeRisk factors predicting distant metastasis (DM) in extrahepatic bile duct cancer (EHBDC) patients treated with curative resection were investigated.
Materials and MethodsMedical records of 1,418 EHBDC patients undergoing curative resection between Jan 2000 and Dec 2015 from 14 institutions were reviewed. After resection, 924 patients (67.6%) were surveilled without adjuvant therapy, 297 (21.7%) were treated with concurrent chemoradiotherapy (CCRT) and 148 (10.8%) with CCRT followed by chemotherapy. To exclude the treatment effect from innate confounders, patients not treated with adjuvant therapy were evaluated.
ResultsAfter a median follow-up of 36.7 months (range, 2.7 to 213.2 months), the 5-year distant metastasis-free survival (DMFS) rate was 57.7%. On multivariate analysis, perihilar or diffuse tumor (hazard ratio [HR], 1.391; p=0.004), poorly differentiated histology (HR, 2.014; p < 0.001), presence of perineural invasion (HR, 1.768; p < 0.001), positive nodal metastasis (HR, 2.670; p < 0.001) and preoperative carbohydrate antigen (CA) 19-9 ≥ 37 U/mL (HR, 1.353; p < 0.001) were significantly associated with inferior DMFS. The DMFS rates significantly differed according to the number of these risk factors. For validation, patients who underwent adjuvant therapy were evaluated. In patients with ≥ 3 factors, additional chemotherapy after CCRT resulted in a superior DMFS compared with CCRT alone (5-year rate, 47.6% vs. 27.7%; p=0.001), but the benefit of additional chemotherapy was not observed in patients with 0-2 risk factors.
IntroductionExtrahepatic bile duct cancer (EHBDC) is a rare malignancy with poor prognosis [1,2]. Complete surgical resection is considered as the only curative treatment modality, however complete resection is often limited because of advanced stage on presentation [3,4]. Further, even after radical resection, the prognosis still remains poor because of frequent recurrences [5,6]. Therefore, attempts to improve the prognosis through adjuvant treatment have been made, but the benefit of adjuvant treatment was not conclusive and the optimal modality was not determined [7-10].
In a previous multicenter retrospective study (Korean Radiation Oncology Group [KROG] 1814), the role of adjuvant radiotherapy (RT) was evaluated in patients treated with curative surgical resection for EHBDC [11]. Although patients in adjuvant RT group had more poor prognostic factors than in no adjuvant group, adjuvant RT significantly improved the overall survival (OS), locoregional recurrence-free survival (LRFS) and disease-free survival. When adjuvant RT group was analyzed separately, concurrent chemoradiotherapy (CCRT) followed by chemotherapy showed greatest benefit for survival outcome.
In this study, we evaluated risk factors predictive for distant metastasis (DM) in EHBDC patients using KROG 1814 data and identified group of patients who might benefit most from adjuvant chemotherapy.
Materials and Methods1. PatientsThe study population was described in detail in the previous publication [11]. Briefly, 1,475 patients undergoing curative surgical resection for EHBDC at 14 institutions between January 2000 and December 2015 were included. And, following patients were further excluded for this study: those receiving RT alone, pathology other than adenocarcinoma and/or follow-up duration less than 6 months.
Following the study protocol approved by the Institutional Review Board of each participating institution, clinical, pathological, and radiological data were obtained. Types of surgery, preoperative carbohydrate antigen (CA) 19-9 level, tumor location, pathologic stage according to the American Joint Committee on Cancer (AJCC) system seventh edition, resection margin status, and perineural invasion (PNI) were recorded. The resection margin was defined as positive if invasive carcinoma or carcinoma in situ was present at the resection margin. Tumor location was divided into three groups as perihilar, distal, or diffuse. Perihilar tumor was defined as a tumor involving main lobar bile duct proximal to the origin of cystic duct. Tumor arising in common bile duct between the origin of cystic duct and the ampulla of Vater were defined as distal, and tumor with involvement of both perihilar and distal location was recorded as diffuse tumor.
To exclude the confounding effect of treatment, risk factors for DM were evaluated in patients who received no adjuvant treatment after surgical resection. The prognostic value of the risk factors was verified in patients undergoing adjuvant treatment.
2. Statistical analysisDM was defined as any recurrence outside the tumor bed or regional lymph nodes. Distant metastasis-free survival (DMFS) was calculated from the date of the surgical resection. Actuarial survival rate was calculated with the Kaplan-Meier method and the differences were verified with the logrank test. Factors with p-value < 0.05 in univariate analysis were included in multivariate analysis using Cox proportional-hazards. All statistical analyses were performed using SPSS software ver. 27.0 (IBM Corp., Armonk, NY).
Results1. Patient characteristicsA flow diagram of patient selection is shown in Fig. 1. Among 1,369 patients analyzed, 924 (67.5%) had surgery alone, 297 (21.7%) received postoperative CCRT and 148 (10.8%) received postoperative CCRT followed by chemotherapy. As for concomitant chemotherapy during radiation, 97.8% of patients (n=435) received fluoropyrimidine-based chemotherapy and 10 patients (2.2%) received gemcitabine-based chemotherapy. In 148 patients undergoing maintenance chemotherapy after CCRT, fluoropyrimidine-based chemotherapy was offered in 95.9% of patients and gemcitabine-based chemotherapy in 4.1%.
Nine hundred twenty-four patients surveilled without adjuvant treatment after surgical resection were evaluated. Patient characteristics are summarized in Table 1. The median age was 68 years (range, 34 to 90 years) and 585 patients (63.3%) were male. Regarding tumor location, 240 patients (26.0%) had perihilar tumors, 621 (67.2%) had distal tumors, and 63 (6.8%) had diffuse involvement. Bile duct resection was performed in 154 patients (16.7%), bile duct resection with hepatectomy in 203 (22.0%), and pancreaticoduodenectomy in 555 (60.1%). Lymph node metastasis was present in 256 patients (27.7%) and the resection margin was negative in 805 (87.1%). One hundred and seventy-seven patients (19.2%) had poorly differentiated tumor and 640 patients (69.3%) had perineural invasion. The CA 19-9 was elevated in 528 patients (57.1%) before surgery.
2. SurvivalMedian follow-up duration was 37.5 months (range, 2.7 to 213.2 months) in all patients and 59.4 months (range, 6.2 to 213.2 months) in surviving patients. The 5-year OS and LRFS rates were 48.7% and 57.2%, respectively. DM developed in 360 patients (39.0%) and the 5-year DMFS was 57.7% (Fig. 2). The most common site of DM was liver (192 patients, 53.3%) followed by peritoneum (113 patients, 31.4%).
3. Risk factors for DMThe results of univariate and multivariate analysis are summarized in Table 2. Univariate analysis showed that perihilar or diffuse location (p=0.001), T3-4 tumor (p=0.002), nodal involvement (p < 0.001), poor differentiation (p < 0.001), presence of PNI (p < 0.001), margin involvement (p < 0.001), and elevated preoperative CA 19-9 (≥ 37 U/mL, p < 0.001) were significantly associated with inferior DMFS. On multivariate analysis which incorporated factors found significant on univariate analysis, tumor location, nodal metastasis, histologic differentiation, PNI, and preoperative CA 19-9 level were found to be significant risk factors for DM. Based on the result of multivariate analysis, patients were stratified according to the number of risk factors. There were 77 patients (9.2%) with no risk factor, 179 (21.4%) with one, 265 (31.7%) with two, 198 (23.7%) with three, 102 (12.2%) with four, and 14 (1.7%) with five, and the DMFS rates were significantly different according to the number of risk factors with 5-year rates of 87.3%, 75.7%, 59.4%, 36.9%, 28.9%, and 23.4%, respectively (Fig. 3).
The prognostic value of risk factors found in the analysis was validated in patients receiving adjuvant treatments. Patients were divided into two groups according to the number of risk factors using 3 as a cutoff. In group with less than three risk factors, no significant difference in DMFS was found between patients treated with CCRT and those treated with CCRT followed by chemotherapy (p=0.754) (Fig. 4A). However, in group with three or more risk factors, patients undergoing additional chemotherapy after CCRT showed superior DMFS compared to those patients receiving CCRT alone (5-year rate, 48.4% vs. 27.6%; p=0.001) (Fig. 4B).
DiscussionIn current study, tumor location, histologic differentiation, PNI, nodal metastasis, and preoperative CA 19-9 level were identified as significant risk factors for DM, and the prognostic value of these factors were verified in patients undergoing adjuvant CCRT or CCRT followed by chemotherapy.
Because of the high recurrence rate in EHBDC treated with surgical resection, the role of adjuvant treatment has been investigated. But, the evidence supporting adjuvant chemotherapy was not conclusive. Several retrospective studies evaluating the role of chemotherapy with or without RT showed conflicting results [12-14]. Due to the rare incidence, randomized studies testing the optimal adjuvant treatment in EHBDC alone were scarce. BCAT trial, only study which compared observation with adjuvant gemcitabine after surgical resection of EHBDC, failed to demonstrate the difference in relapse-free survival (RFS) and OS between the two groups [15]. However, the BILCAP trial, which included all sites of biliary tract cancer, showed the improved outcome with adjuvant chemotherapy. In this study, patients were randomized into observation versus Capecitabine after surgical resection and capecitabine significantly improved outcome in terms of RFS and OS [16]. Based on these results, adjuvant Capecitabine is recommended for surgically resected biliary tract cancer by the American Society of Clinical Oncology [17]. A recent report on the long-term outcome again showed the improved RFS and OS of the Capecitabine group [18]. However, Capecitabine did not affect local recurrence rate even in patients with involved resection margin, resulting in the local recurrence comprising approximately 50% of the total recurrences in those patients. Notably, involved resection margin was a significant adverse risk factor affecting OS along with nodal involvement and poor differentiation. In our previous study, we reported the benefit of adjuvant RT in local control as well as in OS for patients with EHBDC [11]. In line with the previous study, we aimed to identify prognostic factors for DM in patients with EHBDC and also the subgroup who could benefit from additional chemotherapy after CCRT. We found five risk factors for DM and showed the benefit of additional chemotherapy in patients with high-risk features for DM. Therefore, we believe that results from current analysis could provide additional reliable evidence to supporting the role of adjuvant chemotherapy in patients with EHBDC.
Previously, several studies reported the prognostic factors for survival of resected EHBDC patients. Kim et al. [19] evaluated EHBDC patients treated with curative surgical resection followed by adjuvant CCRT. The major pattern of failure was DM. Hilar tumor location, tumor size ≥ 2 cm, lymph node metastasis, and poorly differentiated tumor histology were identified as prognostic factors for DM. Need for intensified chemotherapy was suggested for patient with multiple risk factors. Lim et al. also compared the outcome of CCRT versus CCRT followed by chemotherapy in patients with EHBDC after resection [20]. They identified that CA 19-9 level, histologic differentiation, nodal status, and pattern of adjuvant treatment were prognostic factors for disease-free survival. CCRT followed by chemotherapy significantly prolonged disease-free survival, especially in subgroup of patients with positive resection margin. Although tumor location was not a significant prognostic factor, patients with proximal tumor benefited from additional chemotherapy after CCRT, while those with distal tumor did not. Another study by Im et al. [21] also evaluated EHBDC patients undergoing curative resection. In their results, preoperative and postoperative CA 19-9 level, histologic grade, and nodal status were prognostic factors for DMFS and adjuvant chemotherapy after surgical resection improved DMFS. Ecker et al. [22] identified curatively resected EHBDC patients from the National Cancer Data Base and evaluated the efficacy of adjuvant therapy after propensity score matching. They showed the benefit of adjuvant chemotherapy with or without RT in patients with high-risk features, including advanced T category, nodal metastasis, lymphovascular invasion, and poor differentiation. Based on these results, it is postulated that patients with high risks should be considered for further adjuvant chemotherapy even after curative resection. Because of the retrospective nature of studies and heterogeneity in adjuvant treatment, nominated risk factors were slightly different across the studies. Additionally, except for the study by Kim et al. [19], the treatment outcome evaluated was mainly disease-free survival or OS and the prognostic factors specific for DM were not evaluated. Contrary to the previous studies, current study evaluated only patients who did not receive any adjuvant treatment after surgical resection and focused on the risk factors for DM. Therefore, the risk factors identified in current study could help to identify the patients who could derive the most benefit from adjuvant chemotherapy with minimal confounding effect.
When compared with the historical reports, the OS rate of the surgery-alone group in our study seems to be better. Although the proportion of resection margin involvement, lymph node metastasis, and other adverse risk factors affecting outcomes were quite different across studies, a recent quality assessment-based meta-analysis reported the pooled locoregional recurrence rate, DM rate, and OS rates of the surgery group not receiving adjuvant RT were 52.1% (crude rate), 39.3% (crude rate), and 25.6% (5-year rate), respectively [23]. Better OS of our surgery-alone group might suggest that patients with adverse features were more likely to receive adjuvant CCRT with or without chemotherapy in the present study. Meanwhile, this observation might also suggest that DM risk is significant in the patients of the surgery-alone group.
There are several limitations in this study including innate retrospective design. The decision on surgical methods and adjuvant treatment was at the discretion of servicing physician or surgeon. Thus, inherent selection bias may have been present. Additionally, not all factors were available from the medical records and thus may have been missed. Despite these limitations, current study is the largest study evaluating the risk factors for DM in patients with surgically resected EHBDC.
In conclusion, proximal tumor location, poor differentiation, presence of PNI, lymph node metastasis, and increased CA 19-9 were risk factors for DM in EHBDC patients after curative resection. Subset of patients with these risk factors may benefit from adjuvant chemotherapy.
NotesEthical Statement The Institutional Review Board of each participating institution approved this study (approval number: EUMC 2018-11-050) and the requirement for informed consent was waived due to the retrospective nature of the study. Author Contributions Conceived and designed the analysis: Kim K, Chie EK. Collected the data: Park Y, Kim TH, Yu JI, Jung W, Seong J, Kim WC, Choi JH, Chang AR, Jeong BK, Kim BH, Kim TG, Kim JH, Park HJ, Shin HS, Im JH, Chie EK. Contributed data or analysis tools: Kim TH. Performed the analysis: Park Y. Wrote the paper: Park Y, Kim TH, Kim K, Jung W, Seong J, Kim WC, Choi JH, Chang AR, Jeong BK, Kim BH, Kim TG, Kim JH, Park HJ, Shin HS, Im JH, Chie EK. AcknowledgmentsThis study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C1013797).
Fig. 1.Flow diagram of patient selection. CCRT, concurrent chemoradiotherapy; EHBDC, Extrahepatic bile duct cancer; RT, radiotherapy. 
Fig. 2.Kaplan-Meier curves for overall, locoregional recurrence-free, and distant metastasis-free survival in patients undergoing surgery alone. 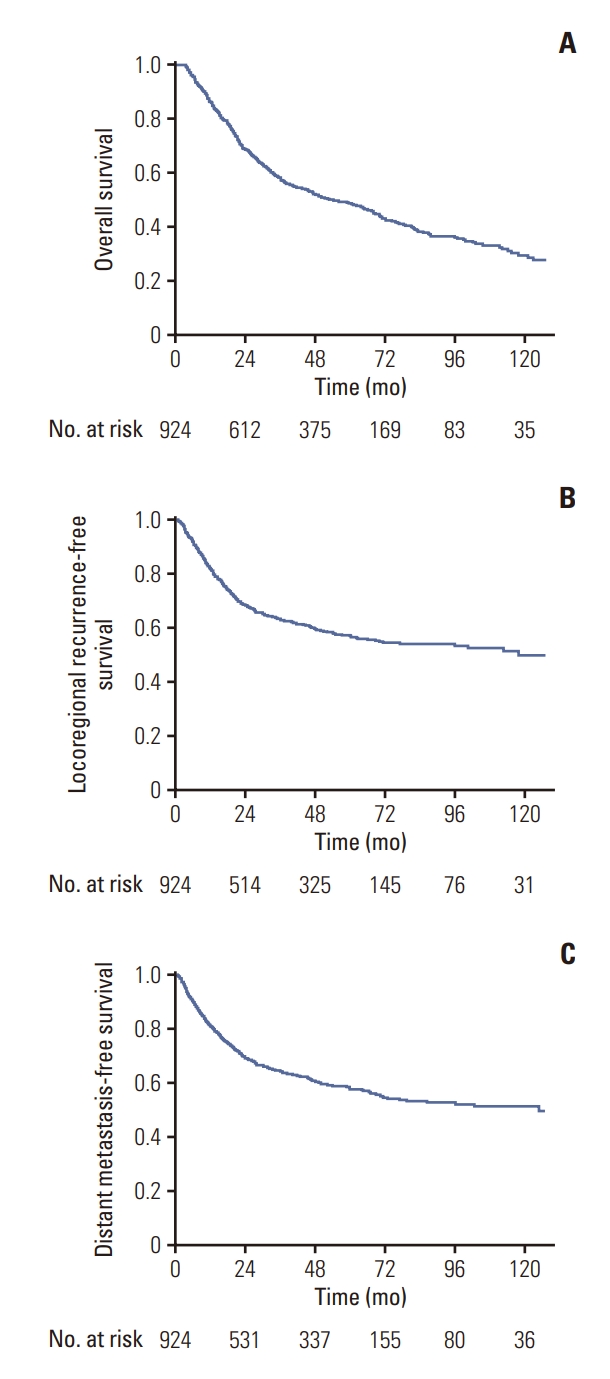
Fig. 3.Kaplan-Meier curves for distant metastasis-free survival according to the number of risk factors in patients undergoing surgery alone. 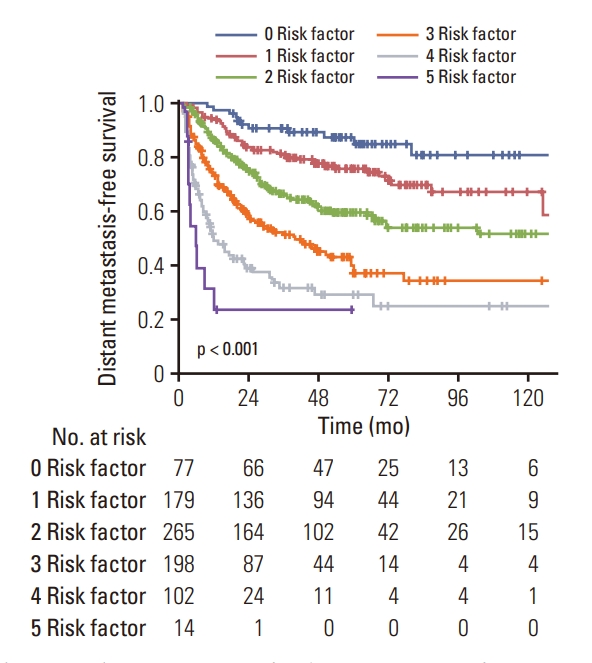
Fig. 4.Kaplan-Meier curves for distant metastasis-free survival according to the receipt of chemotherapy after concurrent chemoradiotherapy (CCRT) in patients with 0-2 risk factors (A) and 3-5 risk factors (B). 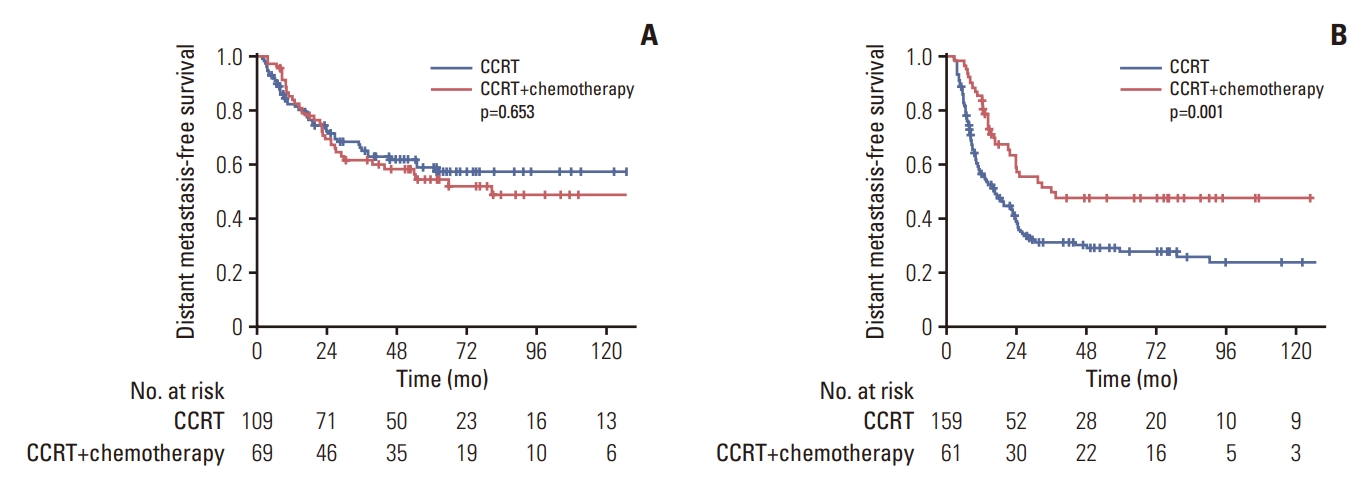
Table 1.Baseline patient and tumor characteristics (n=924) Table 2.Univariate and multivariate analysis for distant metastasis-free survival References1. Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the extrahepatic bile ducts. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70:1498–501.
2. Kopelson G, Galdabini J, Warshaw AL, Gunderson LL. Patterns of failure after curative surgery for extra-hepatic biliary tract carcinoma: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1981;7:413–7.
3. Launois B, Reding R, Lebeau G, Buard JL. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg. 2000;7:128–34.
4. Spolverato G, Bagante F, Ethun CG, Poultsides G, Tran T, Idrees K, et al. Defining the chance of statistical cure among patients with extrahepatic biliary tract cancer. World J Surg. 2017;41:224–31.
5. Jang JY, Kim SW, Park DJ, Ahn YJ, Yoon YS, Choi MG, et al. Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann Surg. 2005;241:77–84.
6. Koo TR, Eom KY, Kim IA, Cho JY, Yoon YS, Hwang DW, et al. Patterns of failure and prognostic factors in resected extrahepatic bile duct cancer: implication for adjuvant radiotherapy. Radiat Oncol J. 2014;32:63–9.
7. Kim TH, Han SS, Park SJ, Lee WJ, Woo SM, Moon SH, et al. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys. 2011;81:e853–9.
8. Kim K, Chie EK, Jang JY, Kim SW, Han SW, Oh DY, et al. Adjuvant chemoradiotherapy after curative resection for extrahepatic bile duct cancer: a long-term single center experience. Am J Clin Oncol. 2012;35:136–40.
9. Rizzo A, Brandi G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2021;15:547–54.
10. Anderson C, Kim R. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: a review of the literature and future directions. Cancer Treat Rev. 2009;35:322–7.
11. Kim K, Yu JI, Jung W, Kim TH, Seong J, Kim WC, et al. Role of adjuvant radiotherapy in extrahepatic bile duct cancer: a multicenter retrospective study (Korean Radiation Oncology Group 18-14). Eur J Cancer. 2021;157:31–9.
12. McNamara MG, Walter T, Horgan AM, Amir E, Cleary S, McKeever EL, et al. Outcome of adjuvant therapy in biliary tract cancers. Am J Clin Oncol. 2015;38:382–7.
13. Krasnick BA, Jin LX, Davidson JT 4th, Sanford DE, Ethun CG, Pawlik TM, et al. Adjuvant therapy is associated with improved survival after curative resection for hilar cholangiocarcinoma: A multi-institution analysis from the U.S. extrahepatic biliary malignancy consortium. J Surg Oncol. 2018;117:363–71.
14. Glazer ES, Liu P, Abdalla EK, Vauthey JN, Curley SA. Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J Gastrointest Surg. 2012;16:1666–71.
15. Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105:192–202.
16. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–73.
17. Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37:1015–27.
18. Bridgewater J, Fletcher P, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Long-term outcomes and exploratory analyses of the randomized phase III BILCAP study. J Clin Oncol. 2022;40:2048–57.
19. Kim K, Chie EK, Jang JY, Kim SW, Han SW, Oh DY, et al. Distant metastasis risk stratification for patients undergoing curative resection followed by adjuvant chemoradiation for extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys. 2012;84:81–7.
20. Lim KH, Oh DY, Chie EK, Jang JY, Im SA, Kim TY, et al. Adjuvant concurrent chemoradiation therapy (CCRT) alone versus CCRT followed by adjuvant chemotherapy: which is better in patients with radically resected extrahepatic biliary tract cancer?: a non-randomized, single center study. BMC Cancer. 2009;9:345.
21. Im JH, Seong J, Lee IJ, Park JS, Yoon DS, Kim KS, et al. Surgery alone versus surgery followed by chemotherapy and radiotherapy in resected extrahepatic bile duct cancer: treatment outcome analysis of 336 patients. Cancer Res Treat. 2016;48:583–95.
|
|
|||||||||||||||||||||||||||||||||||||||