AbstractPurposeAlectinib and brigatinib are second-generation anaplastic lymphoma receptor tyrosine kinases (ALKs) that are widely used as first-line therapy for treating ALK-positive advanced non–small cell lung cancer (NSCLC). Given the lack of a head-to-head comparison of these drugs as first-line therapies, this retrospective observational study aimed to compare the real-world efficacy and safety of alectinib and brigatinib.
Materials and MethodsPatients who received alectinib or brigatinib as the first-line treatment for ALK-positive advanced NSCLC were evaluated for clinical outcomes of objective response rate (ORR), intracranial ORR, time to next treatment (TTNT), progression-free survival (PFS), overall survival (OS), and safety profiles.
ResultsOf 208 patients who received either alectinib or brigatinib as a first-line treatment, 176 received alectinib and 32 received brigatinib. At the data cutoff point, the median follow-up duration was 16.5 months (95% confidence interval [CI], 14.7 to 18.3) in the brigatinib group and 27.5 months (95% CI, 24.6 to 30.4) in the alectinib group. The ORR was 92.5% with alectinib and 93.8% for brigatinib. The intracranial ORR rates were 92.7% (38/41) and 100% (10/10), respectively. The rate of PFS at 12 months was comparable between the alectinib group and the brigatinib groups (84.4% vs. 84.1%, p=0.64), and the median TTNT, PFS, and OS were not reached in either group. Treatment-related adverse events were usually mild, and treatment discontinuation due to adverse events was rare (alectinib 4.5% vs. brigatinib 6.25%).
IntroductionLung cancer is a global cause of mortality, with adenocarcinoma being the most prevalent subtype of non–small cell lung cancer (NSCLC). Over the past few decades, targeted therapy against oncogenic driver kinases (particularly the use of tyrosine kinase inhibitors [TKIs]) has revolutionized the treatment of various solid tumors and has the potential to greatly improve the survival rates and quality of life for such patients [1,2]. Anaplastic lymphoma receptor tyrosine kinase (ALK) TKIs have significantly improved survival and quality of life in patients with various solid tumors harboring ALK oncogenic drivers [3].
ALK gene rearrangement is a well-known oncogenic driver activated in NSCLC, anaplastic large-cell lymphoma, and pediatric neuroblastoma [4,5]. ALK rearrangements exist in approximately 3%–5% of NSCLCs and inappropriately activate the cell signaling pathway, leading to tumor invasion, migration, and uncontrolled cell proliferation [6,7]. The frequency of central nervous system (CNS) metastases in NSCLC with ALK rearrangement is approximately 20%–30%, which is higher than the 10% present in NSCLC at the time of diagnosis. This rate highlights the importance of developing ALK inhibitors with high CNS activity to treat NSCLC [8–10].
The development of ALK inhibitors has rapidly evolved since the introduction of crizotinib, a first-generation ALK inhibitor. Next-generation ALK inhibitors such as ceritinib, alectinib, brigatinib, ensartinib and lorlatinib are designed to have more potent activity to overcome various ALK resistance mutations with high CNS activity [11].
Alectinib is an orally bioavailable, highly selective TKI that inhibits ALK and RET proteins by preventing their phosphorylation. Alectinib has fivefold higher potency for inhibiting ALK than crizotinib and is also effective against ALK mutations that create resistance to crizotinib, such as the L1196M substitution. Alectinib also has very good blood-brain barrier penetration. Brigatinib, another second-generation TKI, demonstrated 12-fold greater potency in inhibiting ALK than crizotinib and was effective against crizotinib-, ceritinib-, and alectinib-resistant ALK mutants. Furthermore, it showed activity against epidermal growth factor receptor (EGFR) mutations, including the T790M mutation [12].
Although these two agents have high systemic and CNS activity with well-tolerable but different safety profiles as first-line therapy, there has been no head-to-head comparison. The objective of this retrospective observational study was to compare the real-world efficacy and safety of alectinib and brigatinib in treatment-naïve advanced NSCLC patients with ALK rearrangement.
Materials and MethodsPrimary metastatic or recurrent NSCLC patients with ALK rearrangement who received either alectinib or brigatinib as first-line therapy at Samsung Medical Center from January 2019 to September 2022 were analyzed in this study. The choice of each drug was made by a physician’s discretion. The inclusion criteria for the study were as follows: (1) adult patients (18 years of age or older) with a pathologically confirmed diagnosis of NSCLC, (2) ALK positivity confirmed with fluorescence in situ hybridization (FISH) or immunohistochemistry (IHC) assay or Ventana ALK (D5F3) IHC, and (3) treated with alectinib or brigatinib as the first-line treatment. This study was approved by our institutional review board (IRB No. 2022-12-061), and the requirement for informed consent was waived due to its retrospective analysis.
Clinical data were collected retrospectively from patient medical records. Clinicopathologic parameters included age, sex, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, histology, methods for ALK confirmation, stage, intracranial involvement, and baseline laboratory findings. Clinical outcomes were evaluated for objective response rate (ORR), intracranial ORR, time to next treatment (TTNT), progression-free survival (PFS), overall survival (OS), and safety.
Alectinib was given at 600 mg twice a day, and brigatinib was administered at 180 mg once daily with a 7-day lead-in of 90 mg once daily. Treatment was administered in 28-day cycles until disease progression, unacceptable toxicity, or death. Continuation of treatment beyond progression was allowed at the physician’s discretion if the clinical benefit could be maintained. Dose reduction or interruption was also performed according to the physician’s judgment. Treatment-related adverse events (TRAEs) were assessed and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) ver. 5.0. The response was evaluated by chest or abdominal/pelvic computed tomography and/or brain magnetic resonance imaging (MRI) every two to three treatment cycles.
The ORR that was assessed by investigators was based on the Response Evaluation Criteria in Solid Tumor (RECIST) ver. 1.1. PFS was defined as the time from the first dose of each treatment until the first occurrence of disease progression or death. The TTNT was measured from the date of initiation of each treatment until the date of initiation of the next line of therapy or death. Patients who remained alive at the analysis cutoff date were censored at that time. The OS was defined as the time between the start of treatment and the occurrence of death from any cause, with patients alive at the time of the last data cutoff and at the time of the last follow-up being censored.
Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS) ver. 27.0 (IBM Corp., Armonk, NY) and R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/). The Kaplan-Meier method was used to estimate the median PFS, TTNT, and OS for each treatment group.
Results1. Patients and treatmentIn total, 208 patients were treated with either alectinib or brigatinib as first-line treatment; 32 patients received brigatinib, which became available in Jan 2021, whereas 176 patients received alectinib, which has been available as a first-line treatment since Jan 2019. The median follow-up duration was 16.5 months (95% confidence interval [CI], 14.7 to 18.3) in the brigatinib group and 27.5 months (95% CI, 24.6 to 30.4) in the alectinib group at the time of data cutoff.
The mean age at initiation of ALK inhibitor was 57.17 years (standard deviation [SD], 12.68), and 54.8% of the patients were female. Most of the patients (64.9%) were never-smokers, 23.4% were ex-smokers, and 11.7% were current smokers. Most patients had an ECOG status of 0–1 (97.6%) and adenocarcinoma histology (97.1%). At baseline, 25.5% of the population had intracranial metastases. Among 53 patients with brain metastasis at the baseline, 35 patients (66.0%) did not receive any local treatment for brain metastasis, and 18 patients received local treatment. Stereotactic radiosurgery (SRS) was conducted for 11 patients (20.8%). Whole brain radiotherapy (WBRT) was conducted for six patients (11.3%), and craniectomy and tumor removal (CRTR) in one patient (1.9%). The FISH or Ventana ALK (D5F3) immunohistochemical assay was used to confirm the presence of ALK rearrangements. Patient characteristics were comparable between the alectinib and brigatinib groups (Table 1).
2. EfficacyThe objective response rate was 92.5% for the alectinib group, and 93.8% for the brigatinib group, respectively. There was no significant difference between the two groups (p=0.53; 95% CI, 0.28 to 6.07) (Table 2). The intracranial ORR was 92.7% (38/41) for the alectinib group and 100% (10/10) for the brigatinib group. CNS response was not observed in two patients in the alectinib group. In the alectinib group, 38 patients had a confirmed ORR, of whom 25 (65.8%) had not received prior local therapy, six (15.8%) had received WBRT, and seven (18.4%) had undergone SRS. Two of the three patients with stable disease as intracranial ORR did not receive local therapy (66.7%), while the other patient received SRS (33.3%). In the alectinib group, response evaluation was not performed for two patients. This was because one patient underwent CRTR surgery and was in a no evidence of disease state with no lesions on the baseline MRI, and the other patient could not perform follow-up MRI due to a worsening condition. Intracranial ORR in brigatinib was observed in 10 patients, of whom seven (70.0%) had not received local therapy, and three (30.0%) received SRS before brigatinib. In the alectinib group, measurable brain metastasis was found in 78.0% of patients (32/39), whereas in the brigatinib group, measurable brain metastasis was found in only half of the patients (5/10) (Table 3). The median PFS was not reached in either the alectinib or the brigatinib group (p=0.64) (Fig. 1). In the alectinib group, the 12-month PFS rate was 84.4%, the 24-month PFS rate was 72.6% and the 36-month PFS rate was 65.2%. In the brigatinib group, the 12-month PFS rate was 84.1%. The median TTNT was also not reached in either group (p=0.39). The probabilities of patients continuing either alectinib or brigatinib for 12 months were 80.8% and 84.1%, respectively (p=0.64) (Fig. 2). Similarly, the 12-month OS rate was 93.3%, the 24-month OS rate was 89.9%, and the 36-month OS rate was 84.6% for the alectinib group. The 12-month OS rate was 95.2% for patients treated with brigatinib (Fig. 3).
A subgroup analysis was conducted to correct any potential biases in time-dependent outcomes, such as PFS, OS, and dose discontinuation, according to follow-up time. This approach was essential due to a more than two-fold difference in the follow-up period and the number of patients, which was attributed to the different time points of introduction of the two drugs in Korea. In the subgroup analysis, only patients who had received their first dose of each drug since January 2021 (when brigatinib was introduced) were included. There were no significant differences in the 68 patients in the alectinib group and 32 patients in the brigatinib group analyzed for median PFS, TTNT, and OS (Fig. 4).
3. SafetyMost adverse events were mild (grade 1–2) and manageable for both drugs. The most common non-hematologic TRAEs were aspartate aminotransferase (AST) elevation (n=134, 76.6%) and alkaline phosphatase elevation (n=130, 74.3%) in the alectinib group, whereas AST elevation (n=25, 78.1%) and alanine transaminase (ALT) elevation (20, 68.8%) were most prevalent in patients treated with brigatinib. The most common hematologic TRAEs were anemia in the alectinib group (n=152, 86.9%) and in the brigatinib group (n=20, 62.5%). Grade 3 or greater TRAEs were observed more frequently in the brigatinib group (n=13, 40.6%) than in the alectinib group (n=48, 27.4%). The most common grade 3 or greater TRAEs in the alectinib group were anemia (n=12, 6.9%) and hyperbilirubinemia (n=10, 5.7%), whereas the most common grade 3 or greater TRAE in the brigatinib group was ALT elevation (n=4, 12.5%) (Tables 4 and 5).
Additionally, 101 patients (57.4%) in the alectinib group required at least one dose level modification due to TRAEs, 27 (15.3%) patients experienced dose interruption, and eight patients (4.5%) required permanent discontinuation of alectinib due to TRAEs. Similarly, in the brigatinib group, five patients (15.6%) required at least one dose level modification due to TRAEs, while six patients (18.8%) experienced dose interruption, and two patients (6.25%) discontinued brigatinib due to TRAEs. No treatment-related deaths were observed in either group (Table 6).
DiscussionThe first evidence of efficacy of alectinib as first-line therapy was provided by a multicenter, single-arm, open-label phase 2 study, which included 46 patients with ALK-rearrangement advanced NSCLC in Japan who had not previously received an ALK inhibitor. In this prior study, an objective response rate of 93.5% was demonstrated, including two complete responses and 41 partial responses [13]. The ALEX trial was followed, which is a randomized, multicenter, open-label, active-controlled study that was conducted on 303 patients with ALK-positive NSCLC who had not received prior systemic therapy for metastatic disease. The patients were randomized 1:1 to receive alectinib (n=152) or crizotinib (n=151). The alectinib group showed an improvement in PFS with a hazard ratio (HR) of 0.53 (95% CI, 0.38 to 0.73; p < 0.001) compared to crizotinib. More importantly, 18 patients (12%) in the alectinib group experienced CNS progression compared with 68 patients (45%) in the crizotinib group (cause-specific HR, 0.16; 95% CI, 0.10 to 0.28; p < 0.001) [14]. Updated results demonstrated that the median PFS was 34.8 months for alectinib and 10.9 months for crizotinib by investigator assessment. The most common TRAEs for alectinib were anemia (22.4%), increased bilirubin level (19.1%), and peripheral edema (18.4%), while adverse events (AEs) related to crizotinib were nausea (49.7%), diarrhea (46.4%), and vomiting (41.1%). The proportion of patients with AEs leading to treatment discontinuation was 13.2% with both alectinib and crizotinib [15].
The ALTA 1L trial, a randomized, open-label, multicenter trial that was conducted in adult patients with advanced ALK-positive NSCLC who had not previously received an ALK-targeted therapy. This trial compared the efficacy of brigatinib and crizotinib as ALK-targeted therapies and randomized 275 patients to receive either brigatinib (n=137) or crizotinib (n=138). ALTA 1L showed a three-year PFS rate in the brigatinib group of 43% compared to 19% in the crizotinib group. Additionally, the median PFS for patients treated with brigatinib was 24.0 months compared with 11.1 months for those treated with crizotinib, with an HR of 0.48 assessed by a blind independent central review (95% CI, 0.35 to 0.66). Moreover, the confirmed rates of intracranial response among patients with measurable lesions were 78% (95% CI, 52 to 94) for brigatinib arm and 29% (95% CI, 11 to 52) for crizotinib arm, respectively, suggesting high CNS activity. The most common TRAEs of brigatinib were diarrhea (58%), increased blood creatine phosphokinase (50%), and coughing (36%), while the TRAEs associated with crizotinib were nausea (59%), diarrhea (56%), and peripheral edema (46%). A dose reduction due to AEs occurred in 44% and 25% of patients in the brigatinib and crizotinib arms, respectively. Treatment was discontinued due to adverse events in 13% vs. 9% of treated patients in the brigatinib and crizotinib arms, respectively [16,17].
The results of the ALEX and ALTA-1L studies have led to the Food and Drug Administration approval of two ALK inhibitors, alectinib and brigatinib, as first-line treatments for ALK-positive NSCLC.
No one has yet been conducted a direct comparison of the efficacy of alectinib and brigatinib as primary treatments for advanced NSCLC patients with ALK rearrangement. Nonetheless, several studies have been conducted to assess and to verify the differences between the two drugs. Carcereny et al., for instance, compared brigatinib and alectinib using computer modeling and analyzed their mechanisms of action and potential resistance to evaluate the relative advantages and disadvantages of both drugs [18]. Ando et al. [19] performed a meta-analysis to investigate the efficiency of first-line alectinib and brigatinib in advanced ALK-positive NSCLC using ALEX, J-ALEX, and ALTA-1L studies, while Reckamp et al. [20] performed a similar approach for ALEX and ALTA-1L studies. Both studies demonstrated that brigatinib and alectinib had a comparable performance but authors emphasized the importance of performing a direct comparison via a randomized controlled study [19,20]. Further, Yu et al. [21] preformed an indirect comparison by reviewing ALEX, J-ALEX, ALESIA, and ALTA-1L studies and found that brigatinib was superior to other ALK inhibitors like crizotinib and ceritinib, and its efficacy and safety profile had comparable performance when compared to second-generation ALK inhibitors.
Our study was trying to compare alectinib with brigatinib as first-line therapies for advanced NSCLC with ALK rearrangement in a real-world setting in Korea, where these two agents were available. In this retrospective analysis, we found that both alectinib and brigatinib were associated with high efficacy. The objective response rate was 92.5% in the alectinib group and 93.8% in the brigatinib group. As expected, CNS metastasis was common in ALK-positive NSCLC [8–10]. Among the 208 patients who enrolled in this study, 53 (25.5%) had measurable brain metastasis at the baseline. The intracranial objective response rate was 92.7% in the alectinib group and 100.0% in the brigatinib group, which is quite promising and is also consistent with the results of previous studies [17,22,23].
With a median follow-up of 27.5 months (95% CI, 24.6 to 30.4) in the alectinib group and 16.5 months (95% CI, 14.7 to 18.3) in the brigatinib group, the rate of PFS at 12 months was comparable (84.4% vs. 84.1%, p=0.97), and the median PFS was not reached in either group. Similar findings were observed for TTNT. Since these two drugs were approved at different time points as first-line therapies, we attempted to conduct a subgroup analysis to minimize preexisting bias. Although patient numbers for subgroup analysis were limited in both groups, we did not find any differences in PFS or TTNT.
Given that ALK-positive advanced NSCLC patients treated with ALK inhibitors showed a long survival time of more than 4 to 5 years, OS data are immature in this analysis, requiring longer follow-up.
In terms of adverse events, both alectinib and brigatinib were well tolerated and associated with mild side effects. The discontinuation rate for each drug was < 10%. In contrast, the dose reduction rate due to adverse events was higher in the alectinib group compared to the brigatinib group (57.4% vs. 15.6%). In the J-ALEX study conducted in Japan, alectinib was given at 300 mg twice a day rather than at 600 mg twice a day, which was the dosage used in the Global ALEX study. The J-ALEX study showed comparable efficacy and a low rate of side effects or a dose reduction rate. As a result, further study will be required to determine whether use of 300 mg of alectinib twice a day has similar efficacy and low toxicity in Asian patients [24]. Early-onset pulmonary events are known to be unique adverse events related to brigatinib with a 3% incidence rate. Although none of the patients developed this adverse event in our study, two patients experienced drug-induced pneumonitis that arose one month and three months after brigatinib treatment began.
This study has several limitations. First, bias existed in the evaluation of the efficacy and side effects due to the retrospective nature of the analysis. Second, the number of patients and follow-up period for each drug varied significantly. Last, given the long survival (more than 5 years) associated with ALK-positive NSCLC, follow-up duration for each drug was short, therefore longer follow-up periods will be required for further evaluation of PFS and OS in these patients.
In conclusion, both alectinib and brigatinib had high clinical efficacy and (consistent with existing pivotal studies) were well tolerated in NSCLC patients with ALK rearrangement as a first-line treatment in the real world.
NotesEthical Statement Patients provided written informed consent to participate in this study and for its publication. This retrospective study was conducted according to the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The Samsung Medical Center IRB approved the study protocol (IRB number 2022-12-061). Fig. 4Subgroup analysis was performed to reduce differences in patient numbers and follow-up periods. Only patients who started alectinib or brigatinib treatment after January 2021 (the time of the brigatinib launch in Korea) were included in this subgroup analysis. Progression-free survival of subgroup (A), time to next treatment of subgroup (B), and overall survival of the subgroup (C). 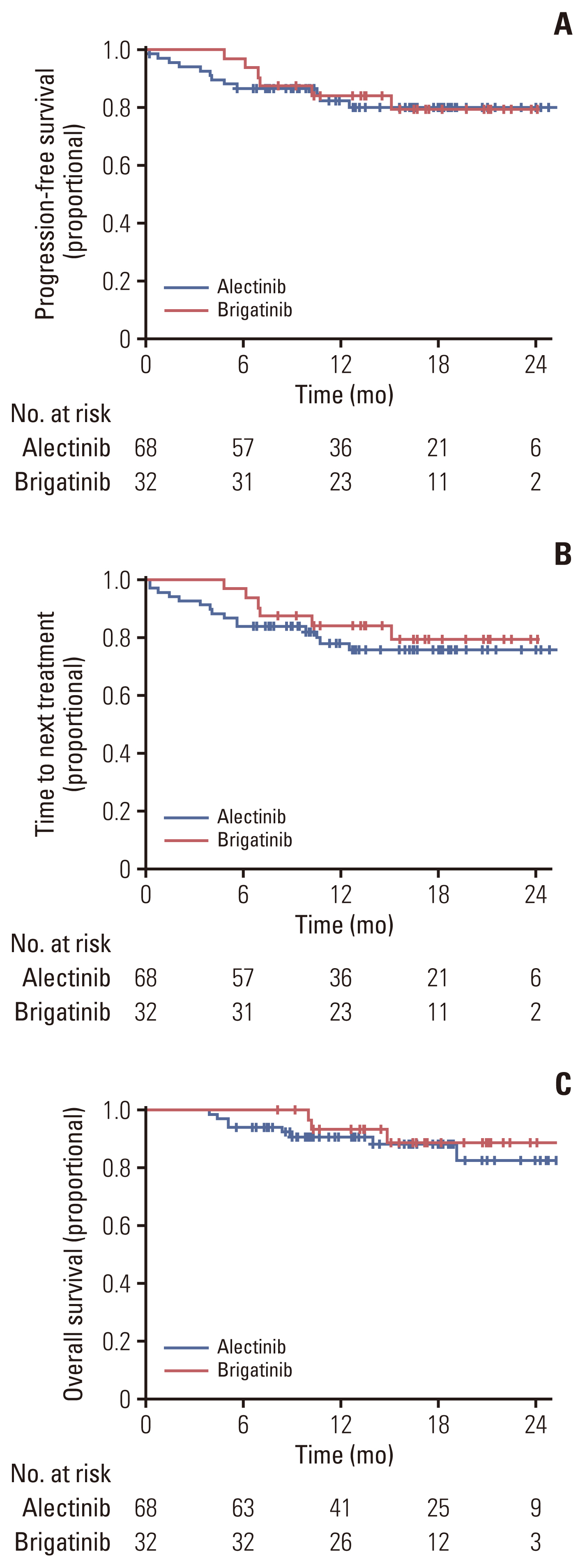
Table 1Baseline characteristics Table 2Best objective response rate Table 3The intracranial best objective response rate Table 4Hematologic adverse events Table 5Non-hematologic adverse events Table 6Dose reduction and interruption References1. Global cancer observatory: cancer today [Internet]. Lyon: International Agency for Research on Cancer; c2020 [cited 2023 May 1]. Available from: https://gco.iarc.fr/today/home
2. Duma N, Santana-Davila R, Molina JR. Non-small cell lungcancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–40.
3. Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, et al. Molecular testing guideline for theselection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol. 2018;36:911–9.
4. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703.
5. Gambacorti-Passerini C, Messa C, Pogliani EM. Crizotinib inanaplastic large-cell lymphoma. N Engl J Med. 2011;364:775–6.
6. Morris TA, Khoo C, Solomon BJ. Targeting ROS1 rearrangements in non-small cell lung cancer: crizotinib and newer generation tyrosine kinase inhibitors. Drugs. 2019;79:1277–86.
7. Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17:637–58.
8. Griesinger F, Roeper J, Pottgen C, Willborn KC, Eberhardt WEE. Brain metastases in ALK-positive NSCLC: time to adjust current treatment algorithms. Oncotarget. 2018;9:35181–94.
9. Johung KL, Yeh N, Desai NB, Williams TM, Lautenschlaeger T, Arvold ND, et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol. 2016;34:123–9.
10. Gainor JF, Ou SH, Logan J, Borges LF, Shaw AT. The central nervous system as a sanctuary site in ALK-positive non-small-cell lung cancer. J Thorac Oncol. 2013;8:1570–3.
11. Sullivan I, Planchard D. ALK inhibitors in non-small cell lung cancer: the latest evidence and developments. Ther Adv Med Oncol. 2016;8:32–47.
12. Zhang S, Anjum R, Squillace R, Nadworny S, Zhou T, Keats J, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res. 2016;22:5527–38.
13. Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14:590–8.
14. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–38.
15. Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14:1233–43.
16. Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379:2027–39.
17. Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Hochmair MJ, et al. Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: final results of phase 3 ALTA-1L trial. J Thorac Oncol. 2021;16:2091–108.
18. Carcereny E, Fernandez-Nistal A, Lopez A, Montoto C, Naves A, Segu-Verges C, et al. Head to head evaluation of second generation ALK inhibitors brigatinib and alectinib as first-line treatment for ALK+ NSCLC using an in silico systems biology-based approach. Oncotarget. 2021;12:316–32.
19. Ando K, Akimoto K, Sato H, Manabe R, Kishino Y, Homma T, et al. Brigatinib and alectinib for ALK rearrangement-positive advanced non-small cell lung cancer with or without central nervous system metastasis: a systematic review and network meta-analysis. Cancers (Basel). 2020;12:942.
20. Reckamp KL, Lin HM, Cranmer H, Wu Y, Zhang P, Walton LJ, et al. Indirect comparisons of brigatinib and alectinib for front-line ALK-positive non-small-cell lung cancer. Future Oncol. 2022;18:2499–510.
21. Yu Y, Zhu F, Zhang W, Lu S. Comparison of efficacy and safety of brigatinib in first-line treatments for patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer: a systematic review and indirect treatment comparison. J Clin Med. 2022;11:2963.
22. Nishio M, Nakagawa K, Mitsudomi T, Yamamoto N, Tanaka T, Kuriki H, et al. Analysis of central nervous system efficacy in the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer. 2018;121:37–40.
|
|
|||||||||||||||||||||||||||||||||||||