AbstractPurposeThis subgroup analysis of the Korean subset of patients in the phase 3 LASER301 trial evaluated the efficacy and safety of lazertinib versus gefitinib as first-line therapy for epidermal growth factor receptor mutated (EGFRm) non–small cell lung cancer (NSCLC).
Materials and MethodsPatients with locally advanced or metastatic EGFRm NSCLC were randomized 1:1 to lazertinib (240 mg/day) or gefitinib (250 mg/day). The primary endpoint was investigator-assessed progression-free survival (PFS).
ResultsIn total, 172 Korean patients were enrolled (lazertinib, n=87; gefitinib, n=85). Baseline characteristics were balanced between the treatment groups. One-third of patients had brain metastases (BM) at baseline. Median PFS was 20.8 months (95% confidence interval [CI], 16.7 to 26.1) for lazertinib and 9.6 months (95% CI, 8.2 to 12.3) for gefitinib (hazard ratio [HR], 0.41; 95% CI, 0.28 to 0.60). This was supported by PFS analysis based on blinded independent central review. Significant PFS benefit with lazertinib was consistently observed across predefined subgroups, including patients with BM (HR, 0.28; 95% CI, 0.15 to 0.53) and those with L858R mutations (HR, 0.36; 95% CI, 0.20 to 0.63). Lazertinib safety data were consistent with its previously reported safety profile. Common adverse events (AEs) in both groups included rash, pruritus, and diarrhoea. Numerically fewer severe AEs and severe treatment–related AEs occurred with lazertinib than gefitinib.
ConclusionConsistent with results for the overall LASER301 population, this analysis showed significant PFS benefit with lazertinib versus gefitinib with comparable safety in Korean patients with untreated EGFRm NSCLC, supporting lazertinib as a new potential treatment option for this patient population.
IntroductionIn Korea, lung cancer is reported to be the most commonly diagnosed cancer, after thyroid cancer, and the leading cause of cancer-related deaths [1–3]. Similar to global disease trends, non–small cell lung cancer (NSCLC), particularly adenocarcinoma, has become the most common type of lung cancer in Korea, replacing squamous cell carcinoma as the dominant histologic subtype since 2011 [2]. NSCLC is associated with various somatic driver mutations in the epidermal growth factor receptor gene (EGFR) and other genes. The most common EGFR activating mutations, deletions in EGFR exon 19 (Ex19del) and the Leu858Arg (L858R) point mutation in exon 21, result in constitutively active EGFR tyrosine kinase signaling, contributing to tumor growth and development [4].
The development of EGFR tyrosine kinase inhibitors (EGFR TKIs) capable of targeting activating EGFR mutations changed the paradigm of care for patients with NSCLC. This has been especially relevant for Korean and other Asian populations, wherein the prevalence of EGFR activating mutations is much higher (30%–60%) than in non-Asian patients (10%–30%) [5]. First- and second-generation TKIs, such as gefitinib, erlotinib, and afatinib, have demonstrated superiority to conventional chemotherapy for patients with previously untreated advanced or metastatic EGFR-mutated (EGFRm) NSCLC. In such patients, EGFR TKI therapy results in high response rates and extended progression-free survival (PFS), with a median PFS (mPFS) of 9–13 months reported in phase 3 trials, compared with 4–6 months on chemotherapy [6–8]. Consequently, EGFR TKI therapy is currently recommended as a standard first-line therapy for EGFRm NSCLC in Korea and other regions. Despite response rates of 70% or more in the first-line setting, disease progression due to acquired resistance typically occurs within 10–14 months of starting EGFR TKI therapy [6–8]. In approximately 50% of cases, this is due to the EGFR Thr790Met (T790M) resistance mutation [4,9].
A lesser degree of benefit with first- or second-generation EGFR TKI therapy has been described in certain patient subgroups, notably those with pre-existing central nervous system (CNS) involvement and those with L858R-positive tumors. Among patients with pre-existing brain metastases (BM), development of further BM on EGFR TKI therapy is common, and is associated with worse outcomes than in patients without prior BM [10,11]. The limited activity of first- or second-generation EGFR TKIs against BM has been attributed to the minimal CNS penetration of these drugs [11]. In addition, clinical trial data indicate less favorable outcomes with EGFR TKI therapy in patients with the L858R mutation than those with Ex19del mutations [7,12–14].
To overcome T790M resistance and address the need for improved first-line treatment options, third-generation EGFR TKIs have been developed that selectively target activating mutant and T790M mutant EGFR while sparing the wild-type form. These third-generation EGFR TKIs include osimertinib (approved in the United States as a first-line therapy in 2018 based on the results of the FLAURA study [14]), and lazertinib, which was approved in Korea in 2021 for the treatment of patients with T790M-positive advanced NSCLC who previously received EGFR TKI therapy [15]. Lazertinib (YH25448, JNJ-73841937) is a brain-penetrant, highly mutant-selective and irreversible third-generation EGFR TKI. Preclinical studies showed lazertinib to have high selectivity for mutant over wild-type EGFR, and excellent CNS penetration, achieving a brain-to-plasma ratio of 0.9 and intracranial tumor-to-brain ratio of 0.7 in animal models [16]. In a phase 1/2 study of patients with EGFRm NSCLC, lazertinib was well tolerated and exhibited promising systemic antitumor activity [17,18].
The LASER301 phase 3 global study (ClinicalTrials.gov ID: NCT04248829) was designed to assess the efficacy and safety of monotherapy with lazertinib compared with gefitinib as a first-line therapy for EGFRm NSCLC [19]. Here, we report efficacy and safety data for the Korean subset of the LASER301 study.
Materials and MethodsLASER301 is an ongoing double-blind, randomized, phase 3 trial comparing the efficacy and safety of lazertinib with that of gefitinib in treatment-naïve patients with NSCLC harboring activating EGFR mutations. This subset analysis assessed efficacy and safety in Korean patients enrolled at 22 sites in Korea.
1. Study oversight and ethicsThe study was approved by the institutional review boards or independent ethics committees of each study site, and was conducted in accordance with the principles expressed in the Declaration of Helsinki, the International Conference on Harmonization/Good Clinical Practice guidelines, and applicable local laws and regulations. Prior to enrollment, written informed consent was obtained from all patients. In situations where consent could not be given by patients, informed consent was obtained from a legally acceptable representative.
2. Study design and treatmentPatients in the LASER301 study were randomly assigned in a 1:1 ratio to receive either lazertinib (240 mg administered orally, once-daily) or gefitinib (250 mg administered orally, once-daily) (S1 Fig.). Randomization was performed centrally using the permuted block technique, stratified by EGFR mutation status (Ex19del or L858R) and race (Asian or non-Asian). A treatment cycle was 21 days. Patients received their assigned treatment until investigator-assessed objective disease progression based on the Response Evaluation Criteria in Solid Tumours (RECIST) ver. 1.1 criteria. Patients could continue to receive their assigned treatment beyond objective disease progression as long as they continued to show clinical benefit, as judged by the investigator. Patients randomized to the gefitinib arm had the option to receive open-label lazertinib following objective disease progression provided they met all the following criteria: disease progression confirmed by blinded, independent central review (BICR); presence of the T790M mutation post-progression, confirmed locally or centrally by plasma or tissue testing prior to unblinding; no intervening anticancer therapies following gefitinib discontinuation. An archival tumor biopsy sample was required to allow central mutation analysis. Further details of the study design, treatments, and assessments are provided in the Supplementary Materials.
3. PatientsEligible patients were ≥ 18 years of age with locally advanced or metastatic NSCLC not amenable to curative surgery or radiotherapy. Patients were treatment-naïve (prior adjuvant and neo-adjuvant therapy for early-stage disease was permitted if completed > 12 months prior to randomization). Local or central confirmation of Ex19del or L858R mutations in a tissue biopsy, either alone or in combination with other EGFR mutations, was required. Patients had to have a World Health Organization performance status score of 0–1 and no clinically significant deterioration over the 2 weeks before randomization. Patients with asymptomatic and stable BM were eligible.
Patients were excluded if they had leptomeningeal metastases, history of interstitial lung disease (ILD), drug-induced ILD, radiation pneumonitis which required steroid treatment or clinically active ILD, or severe or uncontrolled systemic diseases. Patients with cardiovascular disease (e.g., symptomatic chronic heart failure or serious cardiac arrhythmia, myocardial infarction, or unstable angina), electrocardiogram (ECG) abnormalities, or factors that increase the risk of Fridericia’s corrected QT interval (QTc) prolongation or arrhythmic events were excluded. Full inclusion and exclusion criteria for the LASER301 study are provided in the Supplementary Materials.
4. Endpoints and assessmentsThe primary efficacy endpoint was the duration of PFS, defined as the time from randomization until investigator-assessed objective disease progression (according to RECIST v1.1 criteria), or death from any cause in the absence of progression. Assessments were performed every 6 weeks from randomization for the first 18 months, then every 12 weeks until objective disease progression, after which they were followed for survival every 6 weeks.
Secondary efficacy endpoints reported in this subset analysis include the objective response rate (ORR), duration of response (DoR), disease control rate (DCR), overall survival (OS), and pharmacokinetics of lazertinib. OS was defined as the time from randomization until death due to any cause; patients alive at the time of analysis were censored at the last recorded date they were known to be alive. All patients were followed for survival, disease progression (per local standard practice), and any post-study anticancer treatment until loss to follow up, withdrawal of consent, or death (whichever was earlier). Patients who had not progressed or died at the time of analysis (data cutoff) were censored at the time of their last evaluable assessment.
5. SafetyPatient safety was monitored through adverse events (AEs), clinical laboratory parameters, vital signs, ECG parameters and physical examination. Serious AEs and AEs with at least a possibly causal relationship to the study treatment were described for each treatment group and graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) ver. 5.0.
6. Statistical methodsThe first Korean patient was dosed on 13 February 2020, and the data cutoff date was 29 July 2022. The full analysis set (FAS) used for efficacy analyses included all randomized patients. The safety set consisted of patients who received at least one dose of study treatment.
The primary endpoint, PFS, was compared in the two groups using a log-rank test stratified by mutation subtype (Ex19del or L858R) and race (Asian or non-Asian), with the Breslow approach used for handling ties. In the overall LASER301 trial population, to provide 90% power at a two-sided 5% significance level, it was estimated that approximately 207 progression events would be required to detect a hazard ratio (HR) of 0.64, based on an estimated mPFS of 16.5 months for the lazertinib group and 10.5 months for the gefitinib group. Median PFS and 95% confidence intervals (CIs) were estimated using the Kaplan-Meier method, and HRs were estimated using Cox regression models stratified by mutation type and race. The numbers of events and proportions of patients without an event at 6, 12, 18, and 24 months were also summarised for each treatment group.
For the secondary endpoints, ORR and DCR were analyzed using logistic regression models stratified by mutation subtype and race. Similar to PFS, the median time to event and 95% CIs were estimated for DoR and OS using the Kaplan-Meier method. Plasma concentrations of lazertinib were summarised by nominal sampling time. AEs were summarised by treatment group. For this Korean subset analysis, only mutation subtype was used for the stratified analysis. All analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC).
Results1. PatientsThe LASER301 study randomized patients to treatment between February 2020 and September 2021. The Korean subset comprised 172 patients (lazertinib, 87 patients; gefitinib, 85 patients) randomized (Fig. 1). Patients’ baseline demographics and disease characteristics appeared representative of the intended target patient population and were balanced between the treatment groups (Table 1). Almost all patients had metastatic disease. A higher proportion of patients had Ex19del mutations (lazertinib, 57.5%; gefitinib, 56.5%) than L858R mutations (lazertinib, 42.5%; gefitinib, 43.5%). One-third of patients had CNS metastases at study entry: 31 (35.6%) and 25 (29.4%) patients in the lazertinib and gefitinib groups, respectively.
All randomized patients received at least one dose of the assigned study treatment. The median durations of study treatment for the lazertinib and gefitinib groups were 84.1 weeks (range, 0.9 to 126.0 weeks) and 46.0 weeks (range, 0.3 to 120.3 weeks), respectively. In total, 44 patients (50.6%) who received lazertinib and 72 patients (84.7%) who received gefitinib discontinued their assigned treatment in the main study period. The main reasons for treatment discontinuation were progressive disease (24 [27.6%] in the lazertinib group and 58 [68.2%] patients in the gefitinib group) and AEs (lazertinib, 11 [12.6%] patients; gefitinib, 11 [12.9%]). Twenty-six patients (30.6%) in the gefitinib group who discontinued the assigned study treatment crossed over to receive open-label lazertinib after centrally confirmed disease progression, as permitted by the protocol. At data cutoff, 43 patients (49.4%) were still receiving lazertinib and 13 (15.3%) were receiving gefitinib.
2. EfficacyThe FAS for efficacy analyses included all randomized patients. At data cutoff, disease progression or death events had occurred in 45 patients (51.7%) in the lazertinib group and 68 patients (80.0%) in the gefitinib group. The median follow-up for PFS was 23.3 months (interquartile range [IQR], 20.6 to 26.0) for the lazertinib group, and 26.1 months (IQR, 23.3 to 26.1) for the gefitinib group. The HR for disease progression or death favored lazertinib, 0.41 (95% CI, 0.28 to 0.60). Median PFS was significantly longer in the lazertinib group (20.8; 95% CI, 16.7 to 26.1 months) than in the gefitinib group (9.6; 95% CI, 8.2 to 12.3 months) (p < 0.001, stratified log-rank test).
PFS rates consistently favored lazertinib at all timepoints analyzed (6, 12, 18, and 24 months). At 24 months, 45.0% of patients on lazertinib and 12.9% of patients on gefitinib remained progression-free (Table 2). The separation of Kaplan-Meier PFS curves in favor of lazertinib occurred within the first 3 months, and was largely maintained over the follow-up period (Fig. 2). The results of investigator-assessed PFS were supported by the sensitivity analysis of PFS based on BICR (S2 Fig.). In almost all predefined subgroups, the HR for disease progression or death consistently and strongly favored lazertinib (Fig. 3). This included patients with BM at baseline (HR, 0.28; 95% CI, 0.15 to 0.53; p < 0.001) (Fig. 4), and patients with L858R-positive tumors (HR, 0.36; 95% CI, 0.20 to 0.63; p < 0.001) (Fig. 5).
Table 2 summarises the results for the secondary efficacy endpoints. The ORR (lazertinib, 80.5%; gefitinib, 80.0%) and DCR (lazertinib, 97.7%; gefitinib, 94.1%) were similar in the two treatment groups (Table 2). However, the median DoR in the lazertinib group (19.6 months) was twice as long as that in the gefitinib group (9.0 months). Although the best overall response profile was similar in the two treatment groups, the percentage of patients remaining in response was consistently higher at all timepoints in the lazertinib group than the gefitinib group.
At data cutoff, 49 deaths had occurred (28% data maturity), with 24 deaths (27.6%) in the lazertinib group and 25 deaths (29.4%) in the gefitinib group. Since OS data were immature, median OS was not reached in either treatment group (S3 Fig.). The HR for death was 0.91 (95% CI, 0.52 to 1.60; p=0.754). A total of 36 patients (42%) in the gefitinib group received lazertinib post-progression: 26 received it as the per-protocol cross-over treatment, and 10 patients received lazertinib outside of the study as second-line or later-line therapy.
The pharmacokinetic analysis set included patients receiving lazertinib who had at least one measurable concentration collected post-dose. The lazertinib plasma concentration-time profile (geometric mean plasma concentrations at pre-dose, 1–3 hours, and 4–6 hours post-dose on each day 1 of cycle 1, 2, 5, 9, and 13) is shown in S4 Fig. The geometric mean of the trough plasma concentrations of lazertinib ranged from 195.0–211.4 ng/mL and remained similar from cycles 2 to 13.
3. SafetyThe safety analysis set consisted of patients who received at least one dose of study treatment. One or more AEs due to any cause were reported in 87 (100%) of patients on lazertinib and 84 (98.8%) of patients on gefitinib (Table 3). AEs of grade 3 or higher occurred in 34 patients (39.1%) in the lazertinib group and 43 patients (50.6%) in the gefitinib group. Serious AEs occurred in 29 patients (33.3%) in the lazertinib group and 24 patients (28.2%) in the gefitinib group. Eleven patients in each group (lazertinib 12.6%; gefitinib 12.9%) reported AEs leading to permanent treatment discontinuation.
Table 4 summarises treatment-emergent AEs (any cause) that occurred in ≥ 15% of patients in either treatment group. The most commonly reported AEs were paraesthesia in the lazertinib group (52.9% of patients; 8.2% in the gefitinib group), rash (46.0% and 50.6% of patients, respectively), pruritus (43.7% and 35.3% of patients, respectively), and diarrhea (28.7% and 48.2% of patients, respectively) in both groups. ILD was reported in two patients (2.3%) in the lazertinib group and two patients (2.4%) in the gefitinib group, leading to treatment withdrawal in these four patients. No severe (grade ≥ 3) QTc prolongation events were reported for patients in the lazertinib group, compared with two patients (2.4%) in the gefitinib group.
DiscussionAnalyses for the overall LASER301 study demonstrated the superior efficacy of lazertinib as first-line treatment compared with gefitinib, a standard-of-care EGFR TKI for EGFRm NSCLC, with an HR for progression or death of 0.45 (95% CI, 0.34 to 0.58) [19]. Within the Korean subset, the 59% reduction in the risk of disease progression or death with lazertinib was consistent with that in the overall study population, and translated to significantly longer PFS for patients receiving lazertinib (mPFS of 20.8 vs. 9.6 months for the gefitinib group). The Kaplan-Meier PFS curves showed early and clear separation favoring lazertinib over the study period. The analysis of investigator-assessed PFS was supported by the results of BICR assessment. Importantly, PFS benefit with lazertinib was consistently observed across the predefined subgroups, notably for patients with BM, and those with the L858R mutation. Response rates were high, approximately 80% in both treatment arms, though mainly consisting of partial responses. However, the lazertinib group showed a much more durable response, with a median DoR 10 months longer than the gefitinib group.
The results of this subset analysis in Korean patients were consistent with those of the overall study, and with available clinical data for lazertinib in EGFRm NSCLC. The mPFS on lazertinib for the Korean subset (20.8 months) was similar to that reported in the Asian subgroup analysis in the overall LASER301 population (20.6 months) [19]. This is among the longest reported for EGFR TKIs in phase 3 global studies to date, and is consistent with the long mPFS (24.6 months) observed for patients who received lazertinib as the first-line treatment in the phase 1/2 LASER201 study [20]. The mPFS in the gefitinib comparator group was within the range of 9–13 months previously reported in trials involving Asian patients with untreated EGFRm NSCLC [6,21,22]. As previously reported for osimertinib, another EGFR mutant–selective third-generation TKI [14], lazertinib therapy significantly improved PFS compared with standard first-line therapy (gefitinib) in the Korean subset, with durable responses. Subgroup analyses for osimertinib in Asian cohorts showed significantly longer mPFS (16.5–19.1 months) with first-line osimertinib than with standard-of-care TKIs [21,22]. The corresponding HRs for osimertinib versus comparator TKIs ranged from 0.54 (95% CI, 0.41 to 0.72) [22] to 0.61 (95% CI, 0.38 to 0.99) [21].
Standard first-line EGFR TKIs, such as gefitinib and erlotinib, show poor penetration of the blood-brain barrier and limited CNS efficacy [11,23]. It has been estimated that nearly 25% of patients with EGFRm NSCLC have BM at initial diagnosis, with an additional 2-year CNS progression risk of up to 20% [11,24]. In Korea, a higher baseline BM incidence has been reported (38.9% of EGFRm patients with BM at diagnosis [25]), which could be related to the routine use of brain magnetic resonance imaging during lung cancer diagnostic workup. The same study estimated that a further 10% of patients with EGFRm NSCLC could be expected to develop BM by the time of progression on first-line EGFR TKI therapy. Considering the impact of BM on quality of life, health resource utilization and survival, availability of therapies that can improve control of CNS disease is important, especially in the first-line setting. Patients with stable or asymptomatic BM were able to enroll in LASER301. At study entry, around one-third of patients in the Korean subset had BM, which is associated with increased risk of further BM development and poorer outcomes [10]. In these patients, there was a significant reduction in risk of progression or death with lazertinib (HR, 0.28; 95% CI, 0.15 to 0.53), as in patients without baseline BM (HR, 0.46; 95% CI, 0.29 to 0.74). These findings are consistent with preclinical and clinical data for lazertinib [16–18]. The LASER201 phase 1/2 study reported promising intracranial responses to lazertinib in patients with measurable baseline CNS disease [18]. Unlike osimertinib, lazertinib is not a substrate of breast cancer resistance protein and only a weak substrate of multidrug resistance-1 (MDR1/P-glycoprotein), and thus may be minimally affected by these efflux transporters, which reduce CNS penetration of drugs [16]. The BM subgroup results also compare favorably with similar subgroup data for osimertinib, which indicated significant PFS benefit in Asian patients with BMs [22]. CNS-specific progression was not assessed in this Korean subset analysis, but will be addressed in a separate analysis of intracranial disease outcomes in patients with available data.
As reported for the overall LASER301 study population, Korean patients with either of the common activating mutation subtypes showed comparable PFS benefit with lazertinib, with an HR of 0.36 (95% CI, 0.20 to 0.63) in the L858R subgroup, similar to the Ex19del group (HR, 0.43; 95% CI, 0.26 to 0.72). This contrasts with trial data for other EGFR TKIs including gefitinib, erlotinib, and osimertinib, that consistently documented shorter PFS in patients with L858R mutations compared with Ex19del mutations [7,12–14]. Differences in total time on treatment, PFS and OS outcomes by EGFR mutation subtype have also been noted in real-world settings: patients with L858R had poorer outcomes than those with Ex19del mutations [25–27]. The possible mechanisms underlying the greater efficacy of EGFR TKIs in patients with Ex19del versus L858R mutations are not fully understood and may be complex. In-vitro studies indicate that the L858R mutant is less sensitive than Ex19del mutants to EGFR TKIs such as gefitinib and erlotinib [28,29]. Ex19del mutations strongly stabilize EGFR in its active conformation and increase inhibitor binding, whereas the structure-destabilizing L858R mutation is thought to reduce binding affinity and therefore sensitivity to EGFR TKI inhibition. Lazertinib, on the other hand, shows potent in-vitro activity against both L858R and Ex19del (> 90% inhibition relative to vehicle), and against T790M and L858R/T790M mutant kinases [16]. It has been suggested that the less favorable outcomes in patients with the L858R mutation in earlier studies could be due to higher frequencies of co-occurring pre-treatment T790M or other less TKI-sensitive uncommon EGFR mutations. Finally, studies using L858R and Ex19del mutant cell lines indicated mutation-specific patterns of EGFR phosphorylation and downstream signaling; this represents yet another possible explanation for the observed differences in outcomes such as DoR to TKI therapy [27]. With the indications of PFS benefit regardless of mutation subtype, lazertinib monotherapy may be an option for patients with L858R-positive tumors, as an alternative to the concept of EGFR TKI and anti-angiogenic combination therapy that was explored in the RELAY study [12].
Pharmacokinetic analyses showed that the lazertinib plasma concentration-time profile was similar to that previously reported for the 240 mg dose level, with steady state of lazertinib achieved within 22 days of dosing, by day 1 of cycle 2 [17].
The observed safety profile of lazertinib was largely consistent with expectations from earlier clinical studies [17,18]. The frequency of AEs reported in the Korean subset was somewhat higher than in the overall LASER301 population, but the relative frequencies of these AEs were similar to that in the overall population. Since pharmacokinetic exposure in the Korean subset did not differ from that in the overall population, we consider it unlikely that the higher frequency of AEs was due to higher lazertinib exposure. Paraesthesia, rash, and pruritus were the most common treatment-emergent AEs with lazertinib, and were mostly mild or moderate in severity, consistent with the EGFR wild-type-sparing activity of lazertinib. The incidence of any-cause grade ≥ 3 AEs and treatment-related grade ≥ 3 AEs was lower in the lazertinib group than the gefitinib group, mainly due to a higher incidence of liver enzyme (alanine aminotransferase, aspartate aminotransferase) elevation in the latter. Treatment-related discontinuation rates were similar in the two groups, and there were no treatment-related deaths. ILD was reported in two patients in each treatment group. Consistent with safety assessments in previous studies [17,18,30], which showed that lazertinib had no clinically relevant effects on QT interval, no severe QTc prolongation was observed in patients receiving lazertinib. Off-target inhibition of human epidermal growth factor receptor 2 (HER2) has been suggested as a possible underlying mechanism for EGFR TKI–associated cardiotoxicity. In in-vitro studies, lazertinib showed high selectivity for mutant EGFR and negligible inhibition of HER2, which may translate to lower potential for HER2-related cardiotoxicity compared with osimertinib or other EGFR TKIs [30].
1. LimitationsOnly one standard-of-care EGFR TKI, gefitinib, was included as a comparator in this study. However, the results of a network meta-analysis indicate that gefitinib, erlotinib, and afatinib have similar efficacy in the first-line setting [31]. The mPFS in patients treated with lazertinib is significantly longer than that reported for these first- or second-generation EGFR TKIs. At data cutoff, OS data were not mature for the overall study population (28% maturity) or for the Korean subset. It is thus premature to make formal conclusions about whether or not there is a clear survival difference between the two study arms. The interim analysis of OS in the Korean subset indicated a slight numerical improvement favoring lazertinib, consistent with the result for the overall population [19]. The final analysis of OS will be performed after approximately 45 months of survival follow-up from the first patient randomized. The results are expected to confirm whether the PFS advantage with first-line lazertinib treatment could potentially translate to longer survival. Post-progression treatment is a well-known confounding factor influencing OS. Of note, 46% of the patients in the gefitinib group received lazertinib after disease progression, either by post-progression cross-over as permitted by the protocol (26 patients), or as subsequent therapy outside of the trial (10 patients). We acknowledge the possibility that OS differences attributable specifically to the assigned first-line treatment (gefitinib or lazertinib) could be masked due to the high cross-over rate from gefitinib to open-label lazertinib after disease progression.
This subset analysis did not address questions relating to post-progression outcomes, CNS-specific progression, or acquired resistance mechanisms. However, the significant PFS benefit observed in patients with and without pre- existing BM is consistent with published clinical data on the intracranial efficacy of lazertinib [18]. As part of the study procedures, brain scans and samples for cell-free DNA testing are being collected at regular intervals. Analyses of these additional data for the overall LASER301 population are planned to address CNS efficacy and mechanisms of acquired resistance to first-line lazertinib therapy.
Besides PFS and OS, we expect that clinicians’ benefit/risk evaluation will also consider a range of factors such as the underlying EGFR mutation profile, CNS disease, tolerability, and quality of life when selecting a first-line treatment.
In conclusion, this analysis of the Korean subset of the LASER301 study showed clinically meaningful PFS benefit and durable responses with lazertinib treatment, compared with standard-of-care gefitinib. This clinical benefit was consistently documented across patient subgroups, regardless of baseline BM status or EGFR mutation subtype (L858R or Ex19del), as reported for the overall study population. These results support lazertinib as a new potential treatment option for patients with untreated locally advanced or metastatic EGFRm NSCLC in the Korean population.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The LASER301 study was performed in accordance with the principles expressed in the Declaration of Helsinki and International Council for Harmonisation Guidelines on Good Clinical Practice. The study was approved by the relevant Institutional Review Board/Ethics Committee for each study site. IRB approval details for study sites in Korea are provided in the Supplementary Information file. All patients provided written informed consent for study participation. This study was approved by the Institutional Review of Severance Hospital, Yonsei University, (No. 4-2019-1222), Samsung Medical Center (No. SMC 2019-11-036-005), Asan Medical Center (No. 2019-1707), Chungbuk National University (No. CBNUH 2019-11-005-103), National Cancer Center (No. NCC2020-0026), Seoul National University Hospital (No. NCC2020-0026), Seoul National University Bundang Hospital (No. B-2002-597-404), Gachon University Gil Medical Center (No. GCIRB2020-027), Gyeongsang National University Hospital (No. GNUH 2019-11-037-023), SMG-SNU Bora-mae Medical Center (No. 10-2020-13), St. Vincent’s Hospital (No. VC19MDGT0260), Ulsan University Hospital (No. UUH 2019-11-024-063), CHA Bundang Medical Center (No. CHAMC 2019-11-015-043), Haeundae Paik Hospital (No. HPIRB 2019-12-004-077), Korea University Anam Hospital (No. 2020AN0008), Kangbuk Samsung Hospital (No. KBSMC 2019-11-023-060), Bucheon St. Mary’s Hospital (No. HC19MDGT0116), Dongsan Hospital (No. DSMC 2019-12-001-032), Ajou University Hospital (No. AJIRB-MED-CT3-19-484), Yeungnam University Hospital (No. YUMC 2019-11-057-040), Eunpyeong St. Mary’s Hospital (No. PC19MDGT0135), and Seoul St. Mary’s Hospital (KC20MDGT0011). Author Contributions Conceived and designed the analysis: Cho BC, Ahn MJ, Kim DW, Kang JH. Collected the data: Lee KH, Cho BC, Ahn MJ, Lee YG, Lee Y, Lee JS, Kim JH, Min YJ, Lee GW, Lee SS, Lee KH, Ko YH, Shim BY, Kim SW, Shin SW, Choi JH, Kim DW, Cho EK, Park KU, Kim JS, Chun SH, Kang JH. Performed the analysis: Choi S. Wrote the paper: Lee KH, Cho BC, Ahn MJ, Lee YG, Lee Y, Lee JS, Kim JH, Min YJ, Lee GW, Lee SS, Lee KH, Ko YH, Shim BY, Kim SW, Shin SW, Choi JH, Kim DW, Cho EK, Park KU, Kim JS, Chun SH, Wang J, Choi S, Kang JH. Conflicts of Interest Ki Hyeong Lee reports participation in advisory boards and honoraria from BMS, MSD, AstraZeneca, Pfizer, Eli Lilly, Yuhan Corporation, and research funding from Merck outside the submitted work. Byoung Chul Cho has received research funding from MOGAM Institute, LG Chem, Oscotec, Interpark Bio Convergence Corp, GIInnovation, GI-Cell, Abion, Abbvie, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Champions Oncology, CJ bioscience, CJ Blossom Park, Cyrus, Dizal Pharma, Genexine, Janssen, Lilly, MSD, Novartis, Nuvalent, Oncternal, Ono, Regeneron, Dong-A ST, Bridgebio Therapeutics, Yuhan Corporation, ImmuneOncia, Illumina, KANAPH Therapeutics Inc., Therapex, JINTSbio, Hanmi and CHA Bundang Medical Center. He has received consulting fees from Abion, BeiGene, Novartis, AstraZeneca, Boehringer-Ingelheim, Roche, BMS, CJ, CureLogen, Cyrus Therapeutics, Ono Pharmaceutical, Onegene Biotechnology, Yuhan Corporation, Pfizer, Eli Lilly, GI-Cell, Guardant, HK Inno-N, Imnewrun Biosciences Inc., Janssen, Takeda, MSD, Janssen, Medpacto, Blueprint Medicines, RandBio, and Hanmi. He receives royalty from Champions Oncology, Crown Bioscience, Imagen and serves on the advisory board of KANAPH Therapeutics Inc., Bridgebio Therapeutics, Cyrus Therapeutics, Guardant Health, and Oscotech Inc. He was an invited speaker for ASCO, AstraZeneca, Guardant, Roche, ESMO, IASLC, Korean Cancer Association, Korean Society of Medical Oncology, Korean Society of Thyroid-Head and Neck Surgery, Korean Cancer Study Group, Novartis, MSD, The Chinese Thoracic Oncology Society, and Pfizer. He is on the board of directors for Interpark Bio Convergence Corp., and J INTS BIO. He is a founder of the DAAN Biotherapeutics and owns stocks of TheraCanVac Inc., Gencurix Inc., Bridgebio Therapeutics, KANAPH Therapeutics Inc., Cyrus therapeutics, Interpark Bio Convergence Corp., and J INTS BIO. Myung-Ju Ahn reports research funding from AstraZeneca, Lilly, MSD, Merck, Ono Pharmaceutical, Takeda, Yuhan Corporation, Amgen, Pfizer, Novartis, Roche, Alpha-Pharmaceuticals and honoraria from AstraZeneca, Lilly, MSD, Merck, Ono, Takeda, Yuhan Corporation, Amgen, Pfizer, Novartis and Roche. Youngjoo Lee reports consulting fees from Roche, Merck, Yuhan Corporation, and Bayer. Joo-Hang Kim received grants from AstraZeneca, Yuhan Corporation, Roche, Amgen, and BeiGene. Young Joo Min reports research funding from AstraZeneca, MSD, Merck, Ono Pharmaceutical, Yuhan Corporation, AMGEN, Roche and honoraria from AstraZeneca, MSD, Merck, and Ono Pharmaceutical. Jin-Hyuk Choi reports research funding from AstraZeneca, Roche, and Samyang Holdings Korea. Dong-Wan Kim reports research funding to his institution from Alpha Biopharma, Amgen, Astrazeneca/Medimmune, Boehringer-Ingelheim, BMS, Bridgebio Therapeutics, Chong Keun Dang, Daiichi-Sankyo, GSK, Hanmi, InnoN, Janssen, Merck, Merus, Mirati Therapeutics, MSD, Novartis, Ono Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, and Yuhan Corporation. Jin Hyoung Kang reports research funding from AstraZeneca, Ono Pharma Korea, Daiichi Sankyo/UCB Japan, Yuhan Corporation and received honoraria from AstraZeneca, Ono Pharmaceutical, Lilly and Boehringer Ingelheim. He is a consultant for MSD Oncology, AstraZeneca, Ono Pharmaceutical, Boehringer Ingelheim, Yuhan Corporation, Genexine and was invited speaker for Boehringer Ingelheim, Pfizer and Roche. Jangyoung Wang and SeokYoung Choi are employees of Yuhan Corporation. All other authors have no relevant relationships to disclose. The study was sponsored by Yuhan Corporation. The study sponsor funded medical writing and editorial support for the publication. AcknowledgmentsThe authors would like to thank the patients, their families, the investigators, and site staff who participated in this study.
Medical writing support was funded by Yuhan Corporation and was provided by SK Tay and G Toh of Tech Observer Asia Pacific Pte Ltd. (Singapore).
Fig. 2Kaplan-Meier estimates of investigator-assessed progression-free survival (PFS) by treatment group. CI, confidence interval; HR, hazard ratio. 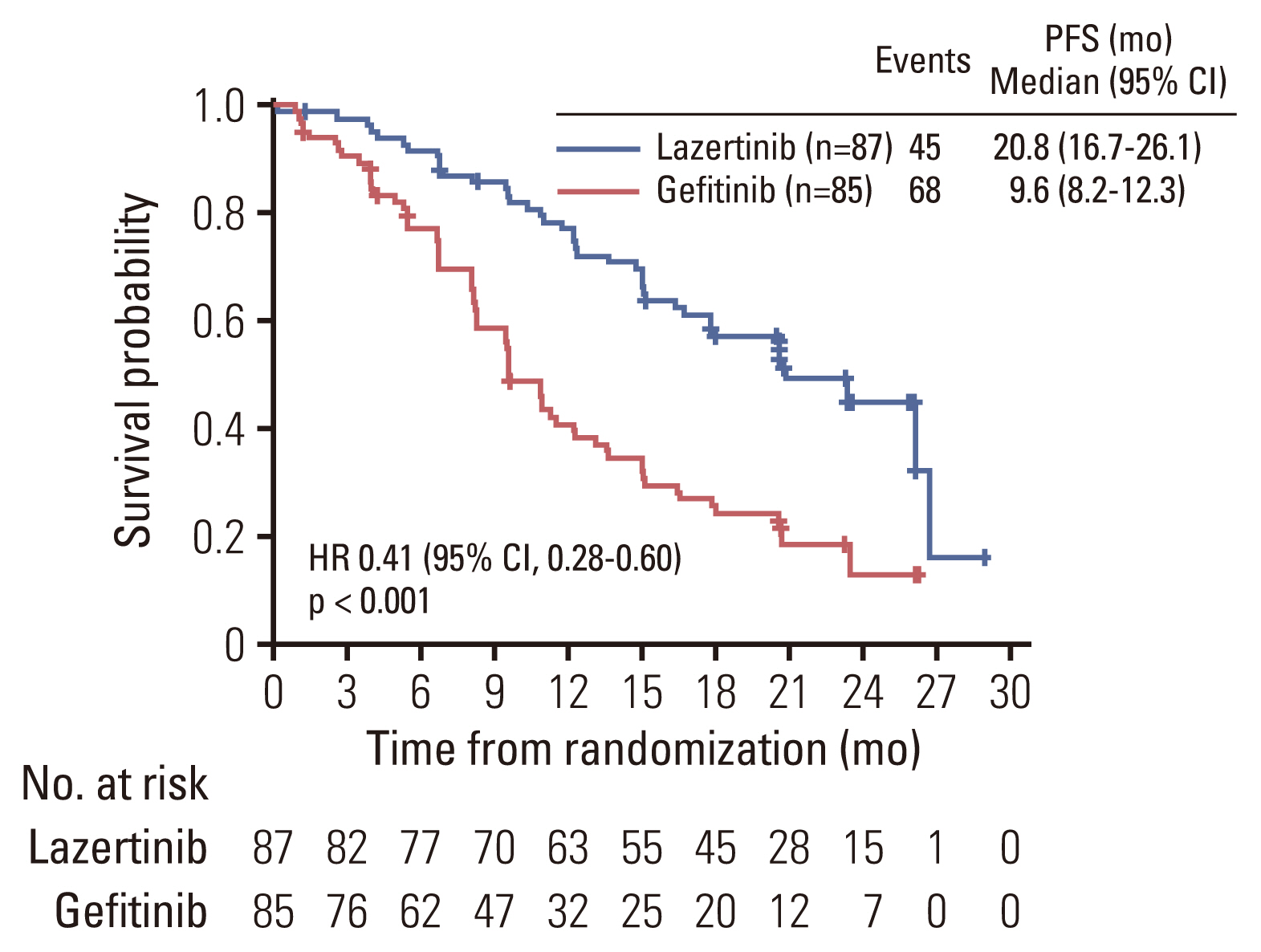
Fig. 3Hazard ratios for progression or death in predefined patient subgroups. Subgroup categories with less than 20 events were excluded from the analysis. CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio; WHO, World Health Organization. 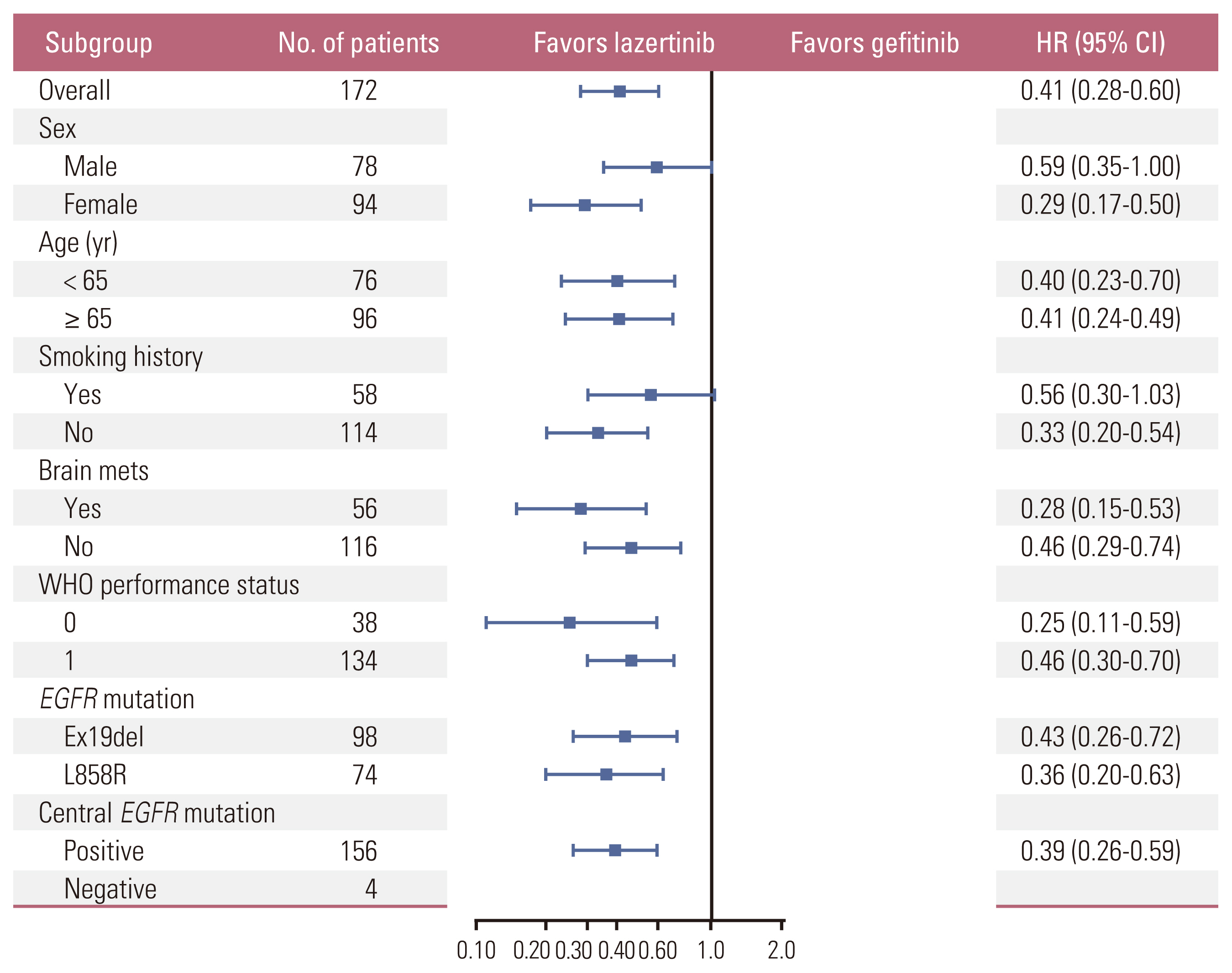
Fig. 4Kaplan-Meier estimates of investigator-assessed progression-free survival (PFS): (A) patients with brain metastases at study entry and (B) patients without brain metastases at study entry. CI, confidence interval; NR, not reached. 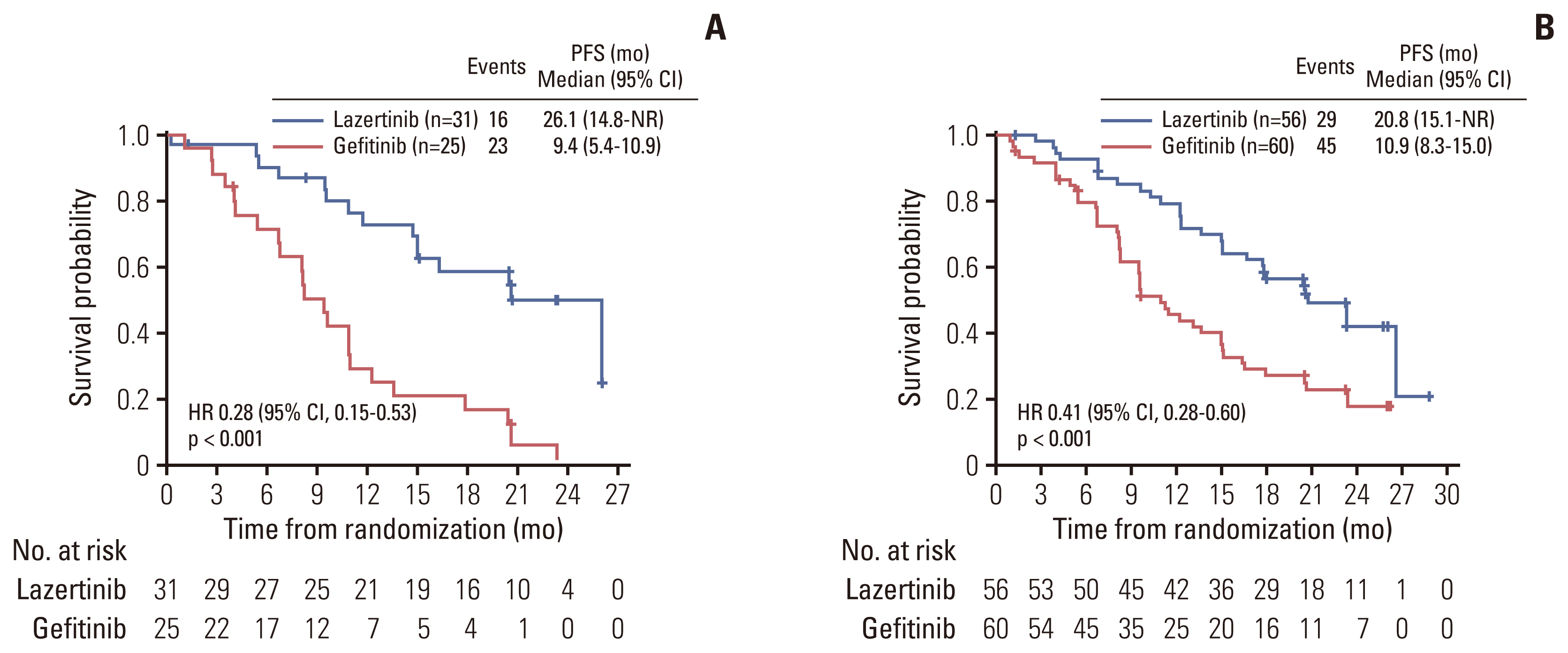
Fig. 5Kaplan-Meier estimates of investigator-assessed progression-free survival (PFS): (A) patients with Ex19del mutation and (B) patients with L858R mutation. CI, confidence interval; NR, not reached. 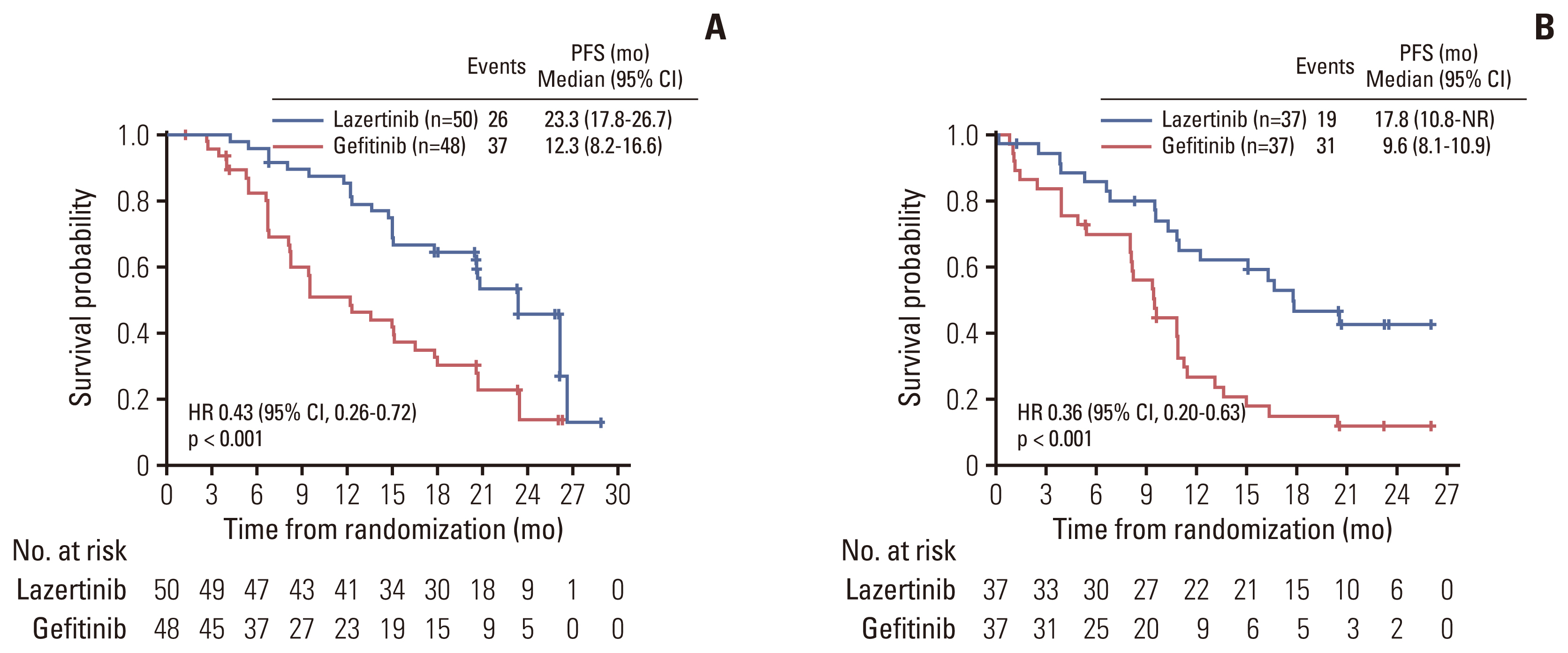
Table 1Patient demographics and clinical characteristics at baseline
Values are presented as number (%). CNS, central nervous system; EGFR, epidermal growth factor receptor gene; SD, standard deviation; WHO, World Health Organization. a) Baseline CNS metastasis status was determined from non–small cell lung cancer history in medical records; no baseline imaging was performed, Table 2Secondary efficacy endpoints
Table 3Overall summary of adverse events (AEs) Table 4Summary of adverse events reported in ≥ 15% of patients in either treatment group References1. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. 2022;54:330–44.
2. Lee JG, Kim HC, Choi CM. Recent trends of lung cancer in Korea. Tuberc Respir Dis. 2021;84:89–95.
3. KOrean Statistical Information Service (KOSIS). Cancer incident cases and incidence rates (1999-2020) [Internet]. Daejeon: Statistics Korea; c2023 [cited 2023 Jun 10]. Available from: https://kosis.kr/
4. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28 (Suppl 1):S24–31.
5. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5:2892–911.
6. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8.
7. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22.
8. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42.
9. Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10–9.
10. Remon J, Besse B. Brain metastases in oncogene-addicted non-small cell lung cancer patients: incidence and treatment. Front Oncol. 2018;8:88.
11. Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88:108–11.
12. Nakagawa K, Nadal E, Garon EB, Nishio M, Seto T, Yamamoto N, et al. RELAY subgroup analyses by EGFR Ex19del and Ex21L858R mutations for ramucirumab plus erlotinib in metastatic non-small cell lung cancer. Clin Cancer Res. 2021;27:5258–71.
13. Sheng M, Wang F, Zhao Y, Li S, Wang X, Shou T, et al. Comparison of clinical outcomes of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations after tyrosine kinase inhibitors treatment: a meta-analysis. Eur J Clin Pharmacol. 2016;72:1–11.
14. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.
15. Ministry of Food and Drug Safety Republic of Korea. LECLAZA (lazertinib): Republic of Korea prescribing information [Internet]. Cheongju: Ministry of Food and Drug Safety; c2021 [cited 2022 Dec 10]. Available from: https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetail?itemSeq=202100467
16. Yun J, Hong MH, Kim SY, Park CW, Kim S, Yun MR, et al. YH25448, an irreversible EGFR-TKI with potent intracranial activity in EGFR mutant non-small cell lung cancer. Clin Cancer Res. 2019;25:2575–87.
17. Ahn MJ, Han JY, Lee KH, Kim SW, Kim DW, Lee YG, et al. Lazertinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: results from the dose escalation and dose expansion parts of a first-in-human, open-label, multicentre, phase 1–2 study. Lancet Oncol. 2019;20:1681–90.
18. Cho BC, Han JY, Kim SW, Lee KH, Cho EK, Lee YG, et al. A phase 1/2 study of lazertinib 240 mg in patients with advanced EGFR T790M-positive NSCLC after previous EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2022;17:558–67.
19. Cho BC, Ahn MJ, Kang JH, Soo RA, Reungwetwattana T, Yang JC, et al. Lazertinib versus gefitinib as first-line treatment in patients with EGFR-mutated advanced non-small cell lung cancer: results from LASER301. J Clin Oncol. 2023;41:4208–17.
20. Cho BC, Han JY, Lee KH, Lee YG, Kim DW, Min YJ, et al. EP08.02-025 lazertinib as a frontline treatment in patients with EGFR mutant advanced non-small cell lung cancer: results from the phase I/II trial. J Thoracic Oncol. 2022;17(9 Suppl):408–9.
21. Ohe Y, Imamura F, Nogami N, Okamoto I, Kurata T, Kato T, et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol. 2019;49:29–36.
22. Cho BC, Chewaskulyong B, Lee KH, Dechaphunkul A, Sriuranpong V, Imamura F, et al. Osimertinib versus standard of care EGFR TKI as first-line treatment in patients with EGFRm advanced NSCLC: FLAURA Asian subset. J Thorac Oncol. 2019;14:99–106.
23. Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399–405.
24. Heon S, Yeap BY, Britt GJ, Costa DB, Rabin MS, Jackman DM, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–82.
25. Jung HA, Hong MH, Lee HW, Lee KH, Kim IH, Min YJ, et al. Totality outcome of afatinib sequential treatment in patients with EGFR mutation-positive non-small cell lung cancer in South Korea (TOAST): Korean Cancer Study Group (KCSG) LU-19–22. Transl Lung Cancer Res. 2022;11:1369–79.
26. Yoon HY, Ryu JS, Sim YS, Kim D, Lee SY, Choi J, et al. Clinical significance of EGFR mutation types in lung adenocarcinoma: a multi-centre Korean study. PLoS One. 2020;15:e0228925.
27. Choi YW, Choi JH. Does the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor differ according to the type of EGFR mutation in non-small cell lung cancer? Korean J Intern Med. 2017;32:422–8.
28. Mulloy R, Ferrand A, Kim Y, Sordella R, Bell DW, Haber DA, et al. Epidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinib. Cancer Res. 2007;67:2325–30.
29. Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, Ross S, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66:8163–71.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||