AbstractPurposeImmunotherapy (IO) plus tyrosine kinase inhibitor (TKI) has become the first-line treatment for advanced renal cell carcinoma, despite the lack of prognostic biomarkers. Cyclin-dependent kinase 5 (CDK5) affects the tumor microenvironment, which may influence the efficacy of TKI+IO.
Materials and MethodsTwo cohorts from our center (Zhongshan Metastatic Renal Cell Carcinoma [ZS-MRCC] cohort, Zhongshan High-risk Localized Renal Cell Carcinoma [ZS-HRRCC] cohort) and one cohort from a clinical trial (JAVELIN-101) were enrolled. The expression of CDK5 of each sample was determined by RNA sequencing. Immune infiltration and T cell function were evaluated by flow cytometry and immunohistochemistry. Response and progression-free survival (PFS) were set as primary endpoints.
ResultsPatients of low CDK5 expression showed higher objective response rate (60.0% vs. 23.3%) and longer PFS in both cohorts (ZS-MRCC cohort, p=0.014; JAVELIN-101 cohort, p=0.040). CDK5 expression was enhanced in non-responders (p < 0.05). In the ZS-HRRCC cohort, CDK5 was associated with decreased tumor-infiltrating CD8+ T cells, which was proved by immunohistochemistry (p < 0.05) and flow cytometry (Spearman’s ρ=−0.49, p < 0.001). In the high CDK5 subgroup, CD8+ T cells revealed a dysfunction phenotype with decreased granzyme B, and more regulatory T cells were identified. A predictive score was further constructed by random forest, involving CDK5 and T cell exhaustion features. The RFscore was also validated in both cohorts. By utilizing the model, more patients might be distinguished from the overall cohort. Additionally, only in the low RFscore did TKI+IO outperform TKI monotherapy.
IntroductionRenal cell carcinoma (RCC) is diagnosed annually in around 430,000 cases worldwide, resulting in approximately 179,000 deaths [1]. Approximately 35% of patients were initially diagnosed with advanced or metastatic renal cell carcinoma (mRCC), while the remaining 65% of patients with localized disease. However, 30% of localized RCC patients will eventually relapse [2]. In the past decade, significant breakthroughs have been made in the treatment of mRCC. Recently, clinical trials of immune checkpoint inhibitor plus vascular endothelial growth factor receptor–tyrosine kinase inhibitor (TKI) for mRCC have exhibited outstanding efficacies [3–5]. Consequently, the European Association of Urology (EAU) Guideline of RCC suggests three TKI+immunotherapy (IO) therapies as standard first-line therapy for mRCC [6]. However, the objective response rate of TKI+IO therapy indicated a poor prognosis for a substantial number of patients. This study aimed to discover prognostic biomarkers for IO/TKI benefit according to multi-omics data of RCC.
Based on published reports, cyclin-dependent kinases (CDKs) participate not only in the cell cycle but also in other essential cellular processes, including gene transcription, insulin secretion, glycogen synthesis, and neuronal functions [7], resulting in tumor development and progression. Hence, we conducted the Kaplan-Meier analysis to screen the CDKs family in both our ZS-MRCC cohort and the TKI+IO subgroup of the JAVELIN Renal 101 cohort (S1 Table). As a result, we initially picked up the CDK5 as a candidate prognostic factor. The proline-directed serine/threonine kinase CDK5 is known as a regulator of neuron function. CDK5 was acting as an independent promising biomarker in lung adenocarcinoma [8], and colorectal cancer patients [9]. Further investigation proved overexpression of CDK5 correlates with poor prognosis, tumor proliferation, migration, and invasion in a range of cancer types, thus playing a vital role in cancer development [10,11]. In recent years, it has been discovered that CDK5 has a remarkable role in cancer immunity. CDK5 activity is essential for interferon γ (IFNG)–mediated cancer immunoevasion [12]. Some studies discovered that the increase of interferon regulatory factor-2 (IRF2) and IRF2-binding protein 2 (IRF2BP2) results in decreased programmed death-ligand 1 (PD-L1) expression on tumor cells in the absence of CDK5 (IRF2BP2). Attenuation of CDK5 expression in a mouse model of medulloblastoma results in robust CD4+ T cell-mediated tumor rejection [13]. CRISPR-Cas9 genome editing system of CDK5 downregulates PD-L1 expression on tumor cells and elicits potent CD8+ T cell- mediated immune responses in the tumor microenvironment (TME) with reduced regulatory T cells (Tregs) [14]. Moreover, CDK5 inhibition increases the level of FBXO22, which leads to the ubiquitination and degradation of PD-L1, hence weakening the immunotherapy sensitivity [15].
In this study, we evaluated CDK5 expression levels of renal carcinoma samples based on RNA-sequencing (RNA-seq) data. The prognostic value CDK5 was assessed for IO/TKI therapy, as well as its correlation with tumor microenvironment components, especially with CD8+ T cells.
Materials and Methods1. Study cohorts and data collection(1) ZS-HRRCC cohortForty-three patients with high-risk localized RCC were enrolled who underwent radical nephrectomy at Zhongshan Hospital, Fudan University from January 2020 to December 2021. Three patients were excluded because of the unavailability of tissue samples or failure to meet sample quality control standards, clinical.
(2) ZS-MRCC cohortTotally, 51 MRCC patients with TKI+IO combination therapy were enrolled from January 2017 to December 2020. Six patients were excluded due to the unavailability of tissue samples or loss of follow-up. Therapeutic response and disease progression were defined by the Response Evaluation Criteria in Solid Tumor (RECIST) ver. 1.1 criteria [16]. Detailed inclusion and exclusion criteria, clinical, pathologic information, treatment response, and survival information of 45 patients were listed in the previous study [17,18].
(3) JAVELIN-101The JAVELIN-101 cohort was disclosed by a clinical trial, enrolling 726 metastatic advanced RCC patients treated by either TKI+IO (avelumab+axitinib, n=354) or TKI monotherapy (sunitinib, n=372) [3].
The Cancer Genome Atlas (TCGA) project enrolled 530 clear cell RCC patients in the The Cancer Genome Atlas Kidney Renal Clear Cell Carcinoma (TCGA-KIRC) cohort (https://xena.ucsc.edu/) [19].
2. RNA-seq and data processingThe MagBeads Total RNA Extraction Kit (MAJORIVD) was used to isolate total RNA. Shanghai Biotechnology Corp. (Shanghai, China) was responsible for the library construction and sequencing. VAHTS Universal V6 RNA-seq Library Prep Kit for Illumina (Vazyme, Nanjing, China) was used for RNA library preparation and NovaSeq 6000 equipment (Illumina, Carlsbad, CA) for sequencing. Sequencing data was further standardized to both fragments per kilobase of transcripts per million mapped reads and read count values.
3. Hematoxylin and eosin staining and immunohistochemistryDetailed primary antibodies for immunohistochemistry (IHC) were described in the previous study [17]. IHC was performed as described before [20]. Slides were scanned with PANNORAMIC 250 Flash III DX (3DHISTECH Ltd., Budapest, Hungary). The densities of targeted cells were calculated as the mean number of cells/mm2. Three researchers were asked to execute the quantification on six randomized fields.
4. Flow cytometryFor surface staining, after blocking Fc receptors, single-cell suspensions and white blood cells were stained with fluorescently labeled membrane marker antibodies for 30 minutes at 4°C. For intracellular staining, proteins were stained with antibodies in Intracellular Fixation & Permeabilization Buffer (Thermo Fisher Scientific, Waltham, MA). Detailed primary antibodies for flow cytometry were used as described before [17]. Flowjo v10.0 was used to analyze BD LSRFortessaTM X-20 (BD Bio-sciences, San Jose, CA) fluorescence-activated cell sorting data (Tree Star, Ashland, OR).
5. In silico approachesAll analysis approaches were performed by R software (https://www.r-project.org/). Cox and Kaplan-Meier analyses were performed by “survival” and “survminer” packages. Random forest model construction was performed by “randomForestSRC” and “ggRandomForests” packages. For volcano plot, the normalized enrichment score cutoff was 1.5.
6. Statistical analysisThe Kruskal-Wallis H test was used to compare continuous variables between groups. The categorical variables were used for the chi-square test. Spearman’s correlation analysis was utilized for quantitative correlation analysis. The cutoff of CDKs family was set at 50%. The cutoff of CDK5 was optimized and set at 33%. All data processing was performed on the R software platform (R Foundation for Statistical Computing, Vienna, Austria).
Results1. CDK5 expression associated with response and prognosis of TKI+IO therapyThe prognostic significance of CDKs families in S1 Table. The latest RCC guidelines recommend TKI+IO combination therapy as the conventional first-line therapy for mRCC patients. However, our ZS-MRCC group showed that mRCC patients exhibited a variety of therapeutic effects (Fig. 1D, E and G). Transcriptomic data proved that expression of CDK5 was enhanced in RCC tissues relative to non-tumorous tissues (TCGA cohort) (p < 0.001) (Fig. 1A). However, we found no significantly different progression-free survival (PFS) between CDK5-low and CDK5-high TxNxM1 RCC patients in the TCGA cohort (data not shown). This may be owing to the fact that their cohorts include patients at all stages and the treatment was different from what it is today. Thus, we assessed the expression of CDK5 in various RCC stages and grades. CDK5 was associated with advanced TNM stage (stage IV) and International Society of Urological Pathology grade in RCC (G4) (Fig. 1B and C). Responders to TKI+IO revealed considerably decreased CDK5 expression (p < 0.05) (Fig. 1D). Meanwhile, the number of patients from low CDK5 expression subgroup responses to TKI+IO were considerably elevated (Fig. 1F and G). Patients with a low CKD5 expression in our ZS-MRCC cohort had a longer PFS (p=0.014) (Fig. 2B) than those with a high CDK5 expression, which was verified in JAVELIN 101 cohort (p=0.040) (Fig. 2C). The cutoff was optimized and set at 33%. Further Kaplan-Meier analysis revealed that patients with a higher CDK5 expression had a worse progression-free survival than those with a lower CDK5 expression, only in the TKI+IO group of the JAVELIN Renal 101 cohort (p=0.04) (Fig. 2C) and in our ZS-MRCC cohort (p=0.014) (Fig. 2B), but not in the TKI monotherapy groups (Fig. 2D). Nevertheless, CDK5 was not predictive factor, as TKI+IO demonstrated better outcome to TKI monotherapy in patients with both high and low CDK5 levels (Fig. 2E and F). Subsequently, both univariate and multivariate Cox regression analysis was performed. Clinical and pathological parameters, including age, sex, histology, International Metastatic RCC Database Consortium (IMDC) group, along with CDK5 expression were incorporated into the Cox regression model. Consequently, we found that CDK5 expression indicated poor prognosis independent of the above clinical and pathological parameters based on PFS (univariate: hazard ratio [HR], 3.199; 95% confidence interval [CI], 1.195 to 8.564; p=0.021; multivariate: HR, 2.841; 95% CI, 1.037 to 7.785; p=0.042) (Fig. 2A). Collectively, these results indicated that the expression of CDK5 could serve as an independent adverse prognosticator for patients with TKI+IO treatment.
2. Association between CDK5 and TME componentsBy hematoxylin and eosin and immunohistochemistry, we identified markers of TME components, including effector cells, regulatory cells, regulatory chemicals, angiogenesis, Ki-67, and polybromo 1 (PBRM1) in our ZS-HRRCC cohort (Fig. 3A). Tumor infiltrating lymphocytes (TILs) did not differ significantly between low and high CDK5 expression groups (Fig. 3B), nor were TILs linked with CDK5 as determined by spearman analysis (data not shown). The number of CD8+ T cells was remarkably decreased in patients with high CDK5 levels (p < 0.05) (Fig. 3C), and was inversely correlated with the expression of CDK5 (Spearman’s ρ=−0.45, p < 0.001, data not shown). Unexpectedly, CD4+ T cells (p=0.720) showed exact opposite trends, compared with CD8+ T cells (p < 0.01) (Fig. 3D).
To further investigate the immunologic state of tumor-infiltrating T cells, we utilized flow cytometry on resected nephrectomy samples from our ZS-HRRCC cohort (Fig. 3E). Consistently, CDK5 was negatively associated with CD8+ T cells (Spearman’s ρ=−0.49, p < 0.001) (Fig. 3F), and positively linked with CD4+ T cells (Spearman’s ρ=0.51, p < 0.001) (Fig. 3G). The CD8+ T cells and CD4+ T cells percentage among total T cells were enhanced in low CDK5 and high CDK5 groups, respectively (p < 0.05) (Fig. 3F and G).
3. T cell dysfunction and exhaustion in high-CDK5 tumorsT cells are crucial anti-tumor mediators that identify and react to tumor-expressed antigens, and they have proved indispensable for cancer immunotherapy. Nonetheless, T cells are not always as anticipated as expected against cancer. T cells attain a dysfunctional or fatigued state defined by sustained expression of inhibitory receptors and a transcriptional state distinct from that of functioning effector or memory T cells. Therefore, flow cytometry was used to evaluate the expression of granzyme B (GZMB) and programmed death-1 (PD-1) on CD8+ and CD4+ T cells in samples from our ZS-HRRCC cohort. Although the amount of CD8+ T cells was reduced in individuals with high CDK5 expression (Fig. 3C and F), a positive connection was identified between GZMB+ CD8+ T cells/total T cells and CDK5 (Spearman’s=−0.51, p < 0.001) (Fig. 4A). Moreover, PD-1+ CD4+ T cells/total T cells were strongly linked with CDK5 (Spearman’s=0.44, p < 0.001) (Fig. 4B). A significant positive connection was identified between Eomesodermin (EOMES; Spearman’s=0.52, p < 0.001) (Fig. 5B), lymphocyte activating gene 3 (LAG3; Spearman’s=0.50, p < 0.001) (Fig. 5C), T-cell immunoreceptor with Ig and ITIM domains (TIGIT; Spearman’s=0.53, p < 0.001) (Fig. 5D), T cell factor 1 (TCF1; Spearman’s=0.30, p=0.060) (Fig. 5E), and CDK5. The expression of IHC markers positive cells were normalized to tumor-infiltrating lymphocytes (Fig. 5A). These results indicated a potential correlation between CDK5 and T cell exhaustion mechanisms.
4. Tregs infiltration in high-CDK5 tumorsImmunosuppressive tumor-infiltrating cells, such as Tregs and macrophages, are putative regulatory mechanisms for T cell exhaustion. The relationship between CDK5 and Treg and macrophage infiltration was examined. There was a positive correlation between Tregs (CD4(+) CD25(+) CD127(−/low) cells) and CDK5 levels (evaluated by flow cytometry, Spearman’s ρ=0.38, p=0.02) (Fig. 4C), which was confirmed by formalin-fixed paraffin-embedded tissues (FoxP3 evaluated by IHC, Spearman’s ρ=0.30, p=0.06) (Fig. 4D). Probably due to the relatively limited cohort size, the p-value of IHC samples was close but not statistically significant. Macrophage infiltration showed no correlation. Macrophages could be polarized into M1- or M2-macrophages under specific TME conditions. Therefore, immunohistochemistry was used to detect M1- or M2-macrophages in the ZS-HRCC cohort. Neither M1 nor M2 macrophages exhibited significant changes in samples with high levels of CDK5. Tregs inhibit the activity of cytotoxic T cells in the TME by the production of transforming growth factor β (TGF-β) [21]. TGF would achieve this by inhibiting the expression of cytolytic gene products, such as granzymes B (GZMB) and K, perforin, FasL, and IFN-γ, from cytotoxic T cells. Spearman analysis showed that the expression of TGF-β was associated with CDK5 expression (Fig. 4E). Besides, through Gene Set Enrichment Analysis, we found that negative regulation of leukocyte proliferation, Tregs differentiation, negative regulation of leukocyte mediated immunity, and negative regulation of T cell–mediated immunity pathways were enriched in high-CDK5 samples (Fig. 4F).
5. Correlation between CDK5 and somatic mutations in RCCA summary of the chromosomal mutations ranked by CDK5 expression for the JAVELIN-101 cohort was compiled (Fig. 4G). Frequent mutations in localized RCC were observed in advanced RCC, including VHL (von Hippel-Lindau tumor suppressor, 55%), PBRM1 (32%), and SETD2 (SET domain containing 2, histone lysine methyltransferase, 25%). Only mammalian target of rapamycin (mTOR) mutation demonstrated a significant relationship with CDK5 expression (p < 0.05) (Fig. 4G).
6. Risk model construction and contribution of componentsThe latest EAU Guidelines for RCC recommended TKI+IO combinations as standard first-line therapy and the PFS of the JAVELIN 101 clinical trial suggested that TKI+IO is a preferred option for mRCC patients. However, the therapeutic benefits of TKI+IO varied by individual. Typically, there is always a portion of patients who responded unfavorably to TKI+IO therapy. Thus, it is necessary to build a model that can determine which subgroup responds best or worse to TKI+IO treatment. Random forest, one of the most prominent machine learning algorithms, was implemented. The expression of CDK5, PD-1, PD-L1, CD8A, CD4, GZMB, and GZMK were enrolled as the parameters for model construction. The contribution of each parameter to the final model was further investigated (Fig. 6A). The predictive value of our model was subsequently validated by Kaplan-Meier. The cutoff was set at 75%. The results indicate that patients with a low signature score in our model have a longer PFS when treated with TKI+IO combination therapy (p < 0.001) (Fig. 6C). This model was subsequently verified in our ZS-MRCC cohort (p=0.002) (Fig. 6D).
Furthermore, TKI monotherapy is an alternative first-line treatment for mRCC patients. Application of our model in mRCC to PFS data from JAVELIN Renal 101 showed in the low RFscore arm (≤ 75% signature score) TKI+IO combination therapy was associated with a trend toward longer PFS (HR, 0.55; 95% CI, 0.43 to 0.706; p=0.009) (Fig. 6B), whereas in the high RFscore TKI+IO and TKI alone did not differentiate PFS (HR, 1.077; 95% CI, 0.720 to 1.610; p=0.719). The random forest model showed a better function in predicting the prognosis of TKI+IO against TKI monotherapy for mRCC patients. Ultimately, Kaplan-Meier validated the predictive value of our model (Fig. 6E).
DiscussionFrom the previous results of our center, patients with high CDK5 expression had no statistically significant trend toward better OS than those with low CDK5 expression [22]. It was consistent with our Kaplan-Meier analysis of both entire cohort or M1 patients from TCGA-KIRC cohort. It is possible that the majority of cases enrolled were at an early stage in our previous report. In reality, the most difficult aspect of RCC treatment is the patients with advanced diseases. Firstly, we demonstrated that CDK5 expression serves as a therapeutic prognosticator for patients with mRCC receiving TKI+IO therapy.
Despite the fact that a portion of patients may benefit from TKI+IO treatment, efficiency, and drug resistance remain major challenges. Studies found that tumor-infiltrating cells, as well as cancer cells, are involved in the process of resistance to cancer treatment. However, little is known about the underlying mechanics of these events. Immune responses and immunosurveillance of cancer cells are essential for tumor development and treatment. Immune contexture, which is characterized by the density, composition, function status, and immune cell infiltration, may be a good determinant of tumor growth and therapeutic response.
Extensive gene, mRNA, and protein-level data support a function for CDK5 in human malignancies as well as its potential as a biomarker for prognosis of cancer. In non–small cell lung cancer, breast, brain, and cancer patient tissues, elevated CDK5 expression associated with advanced cancer stages, lymph node metastasis, and poor 5-year OS survival, while metastatic-free samples showed lower CDK5 levels [23–27]. Functionally, CDK5 may contribute to oncogenesis via overlapping or common pathways, particularly those associated with the cell cycle and proliferation. Through phosphorylating retinoblastoma (Rb), CDK5 regulates the Rb/E2F pathway [28]. CDK5 has also been linked to the deregulation of signal transducer and activator of transcription 3 (STAT3) in cancer cells [29], and is able to phosphorylate the transcription factor androgen receptor in prostate cancer [30]. In breast cancer cells, CDK5 contributed to TGF-β1–induced epithelial-mesenchymal transition in breast cancer cells. TGF-β1 increased the expression of CDK5 and p35, while CDK5 knockdown prevented TGF-β1–induced epithelial-mesenchymal transition. Meanwhile, CDK5 overexpression indicated potential synergy with TGF-β1 in driving epithelial-mesenchymal transition [24]. In lung cancer, blocking CDK5 with inhibitors, siRNA, or the CRISPR-Cas9 system has provided a significant therapeutic advantage, suggesting that CDK5 affects tumor suppressor genes, carcinogenesis, cytoskeletal remodeling, and immunological checkpoints [31].
In medulloblastoma, CDK5 deficiency downregulates PD-L1 by extending the half-life of the IRF2/IRF2BP2 repressor complex, hence promoting T cell responsiveness in medulloblastoma, melanoma, and breast malignancies. Moreover, immunotherapy in combined with CDK5 deletion enhanced anti-tumor immunity [13,14]. Moreover, CDK5 and PD-L1 mRNA co-occurred and were elevated in lung adenocarcinoma, according to TCGA transcriptomic data sets [13]. CDK5 deficiency can increase PD-L1–induced CD4+ T cells mediated cancer cell death. The disruption of CDK5 activity decreases the production of IL-2 via increasing the activity of histone deacetylases and inhibits the binding of STAT3 to the Foxp3 gene promoter, which increased the population of CD8+ T cells while Tregs was decreased [14,32,33]. The abnormal activation of CDK5 is correlated with the progression of triple-negative breast cancer (TNBC). CDK5 modulates the E3 ubiquitin ligase activity of peroxisome proliferator-activated receptor γ and directly protects ESRP1 from ubiquitin-dependent proteolysis, hence enhancing the stemness of TNBC cells. Reducing stemness transformation reverses immunosuppressive TME and improves anti–PD-1 treatment efficacy [34]. In pancreatic cancer, the CDK1/2/5 inhibitor dinaciclib reverses IFNG-mediated adaptive tumor immune resistance. This immunochemotherapeutic strategy increases tumor cell apoptosis and boosts CD8+ T cell-dependent anti-tumor immunity, in many animal models of pancreatic cancer [12]. Additionally, in mouse model of lung cancer, disruption of CDK5 increases the number of CD3+, CD4+, and CD8+ T cells in the spleen and decreases PD-1 expression in CD4+ and CD8+ T cells [35]. Consequently, we questioned whether CDK5 regulates TKI+IO sensitivity in RCC by affecting TME components and T cell functions.
In the present study, we first investigated the TME of mRCC via RNA-seq, flow cytometry, and IHC, and verified the presence of T cell dysfunction within tumor tissues. In our ZS-HRRCC cohort, CDK5 expression was negatively associated with CD8+ T cells (Fig. 3C and F), and positively linked with CD4+ T cells (Fig. 3D and G). Interestingly, although the amount of CD8+ T cells was reduced in individuals with high CDK5 expression, a strong positive connection was identified between either GZMB+ CD8+ T cells/total T cells or PD-1+ CD4+ T cells/total T cells, and CDK5 expression (Fig. 4A and B). Moreover, there was a positive correlation between Tregs and CDK5 levels, which was confirmed by flow cytometry (Fig. 4C and D).
Tregs are a subpopulation of immunosuppressive CD4+ T cells. In established tumors, however, immunosuppressive crosstalk between cancer cells and Tregs suppresses the function of critical effector cells. Expression of CTLA4 by Tregs may also inhibit the suppressive action of CD8+ T cells, hence initiating immunosuppressive activity in the TME [36]. Through the synthesis of adenosine, cancer cells additionally stimulate the activity of myeloid-derived suppressor cells, which then secrete TGF-β for further improvement of Treg immunosuppressive activity [37]. We revealed that CDK5 may regulate T cell exhaustion, either directly or via Tregs, which may result in adverse clinical effects.
In the present study, we identified the CDK5 expression as a novel prognostic factor of PFS for TKI+IO combinations in advanced renal cell carcinoma. Moreover, CDK5 was associated with CD8+ and CD4+ infiltration and T-cell exhaustion.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The study followed the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University (B2021-119). Informed consent was obtained from each participant. AcknowledgmentsWe thank those authors who released and shared their datasets on the TCGA databases and Javelin Renal 101 clinical trial. This study was funded by grants from National Natural Science Foundation of China (81700660, 81902898, 81772696, 81974393), Shanghai Sailing Program (19YF1407900), and Experimental Animal Project of Shanghai Science and Technology Commission (19140905200). All these study sponsors have no roles in the study design, in the collection, analysis, and in the interpretation of data.
Fig. 1Cyclin-dependent kinase 5 (CDK5) related with resistance to tyrosine kinase inhibitor (TKI) plus immunotherapy (IO) combination therapy in renal cell carcinoma (RCC). (A) Expression of CDK5 in RCC and peritumor tissues. p-values, Kruskal-Wallis H test. (B, C) Association between CDK5 and TNM stage/International Society of Urological Pathology (ISUP) grade in RCC. p-values, Kruskal-Wallis H test. (D) Expression of CDK5 between responders and non-responders of TKI+IO combination therapy in the Zhongshan Metastatic Renal Cell Carcinoma (ZS-MRCC) cohort. p-values, Kruskal-Wallis H test. (E, F) Therapeutic response (E) and representative chest computed tomography (F) according to CDK5 in the ZS-MRCC cohort under TKI+IO combination therapy. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease. (G) Tumor best percentage change from baseline and CDK5 expression in our ZS-MRCC cohort of TKI+IO combination therapy. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant. 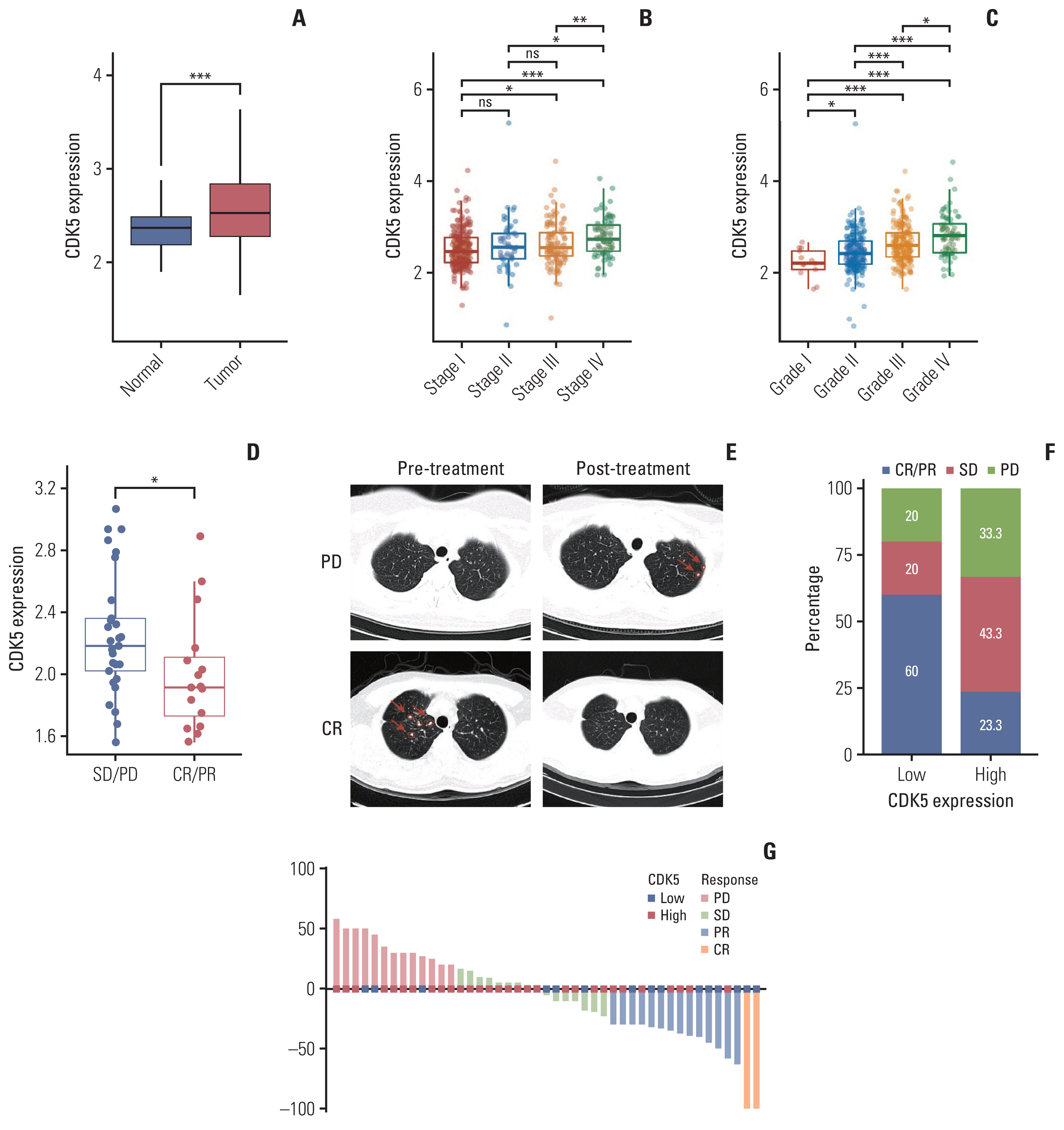
Fig. 2Cyclin-dependent kinase 5 (CDK5) related with prognosis of tyrosine kinase inhibitor (TKI) plus immunotherapy (IO) combination therapy in renal cell carcinoma. (A) Univariate and multivariate Cox regression model was used to calculate hazard ratio (HR) and 95% confidence interval (CI). HR < 1 indicates better survival. The cutoff of CDK5 expression was 33%. (B, C) Progression-free survival after TKI+IO therapy according to CDK5 in the ZS-MRCC cohort (B) and TKI+IO subgroup of JAVELIN 101 cohort (C). cc, clear cell; IMDC, International Metastatic RCC Database Consortium; ZS-MRCC, Zhongshan Metastatic Renal Cell Carcinoma. TKI subgroup of JAVELIN 101 cohort (D). Progression-free survival after TKI+IO or TKI therapy in high-CDK5 (E) and low-CDK5 subgroup (F) of JAVELIN 101 cohort. p-value, Kaplan-Meier analysis, and log-rank test. 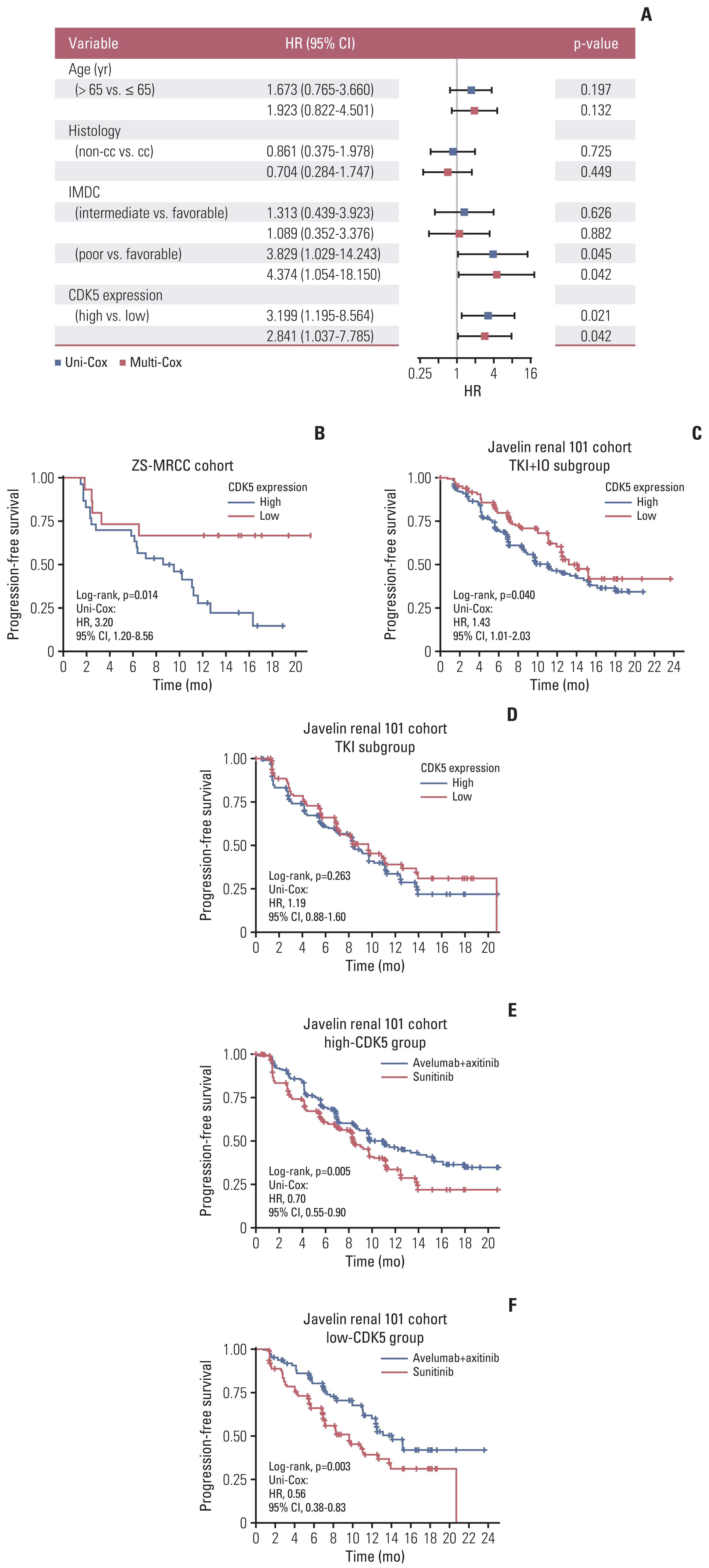
Fig. 3Relationship between cyclin-dependent kinase 5 (CDK5) and tumor microenvironment in renal cell carcinoma. (A) Heatmap displaying tumor microenvironment components ranked by CDK5 in the Zhongshan High-risk Localized Renal Cell Carcinoma (ZS-HRRCC) cohort. (B–D) Representative images and quantification of tumor-infiltrating lymphocytes (TILs) (B), CD8+ T cells (C), and CD4+ T cells (D) sorted by CDK5 expression. α-SMA, α-smooth muscle actin; GZMB, granzyme B; IFN γ, interferon γ; NK, natual killer; PBRM1, polybromo 1; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; Tresg, regulatory T cells. p-values, Kruskal-Wallis H test. (E–G) Representative images of flow cytometry and the association between CD8+ T cells (F) or CD4+ T cells (G), and CDK5 expression in the ZS-HRRCC cohort. ρ and p-values, Spearman’s rank-order correlation. *p < 0.05, **p < 0.01; ns, not significant. 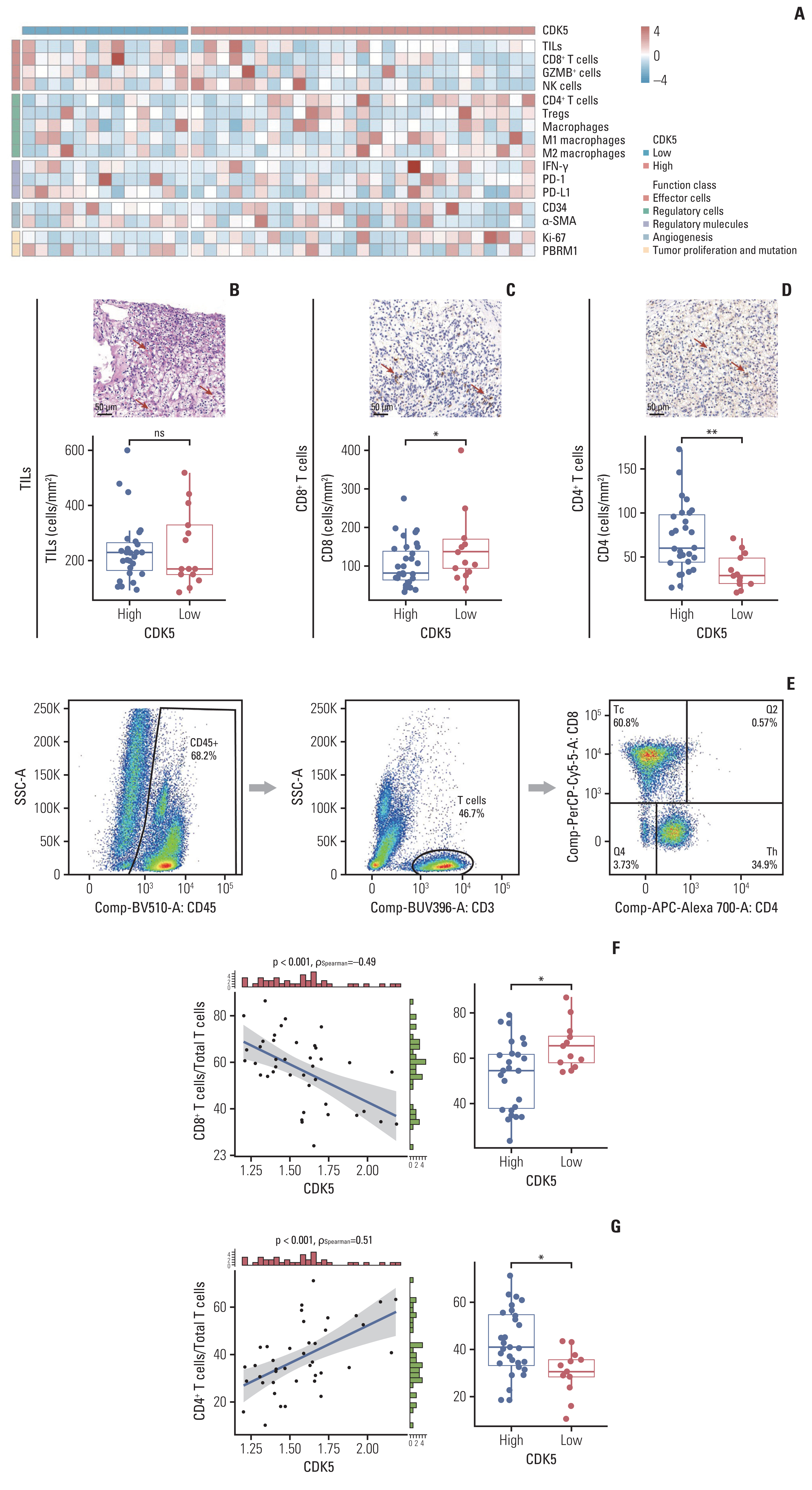
Fig. 4Cyclin-dependent kinase 5 (CDK5) is associated with T cell dysfunction and regulatory T cells (Tregs) infiltration in renal cell carcinoma (RCC). (A–D) Gating strategy of granzyme B (GZMB) CD8+ T cells (A), GZMB+CD8+ T cells (B), and Tregs (C), and their association with CDK5 in Zhongshan High-risk Localized Renal Cell Carcinoma (ZS-HRRCC) cohort by flow cytometry. ρ and p-values, Spearman’s rank-order correlation. (D) Representative images and quantification of Tregs by immunohistochemistry. ρ and p-values, Spearman’s rank-order correlation. (E) Association between transforming growth factor β1 (TGF-β1) expression and CDK5 in the The Cancer Genome Atlas Kidney Renal Clear Cell Carcinoma (TCGA-KIRC) cohort. ρ and p-values, Spearman’s rank-order correlation. (F) Volcano plot of Gene Set Enrichment Analysis of Gene Ontology pathways between high and low CDK5 samples. (G) Waterfall plot displaying genomic mutations ranked by CDK5 expression in the JAVELIN-101 cohort. p-values, chi-square test. *p < 0.05. ARID1A, AT-Rich Interaction Domain 1A; ATM, ataxia-telangiectasia mutated; ATR, ataxia telangiectasia and Rad3-related; BAP1, BRCA1 associated protein 1; HRR, homologous recombination repair; MLH, MutL homolog; MSH, MutS homolog; mTOR, mammalian target of rapamycin; PBRM1, polybromo 1; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha; PTEN, phosphatase and tensin homolog; RICTOR, RPTOR independent companion of MTOR complex 2; SETD2, SET domain containing 2, histone lysine methyltransferase; VHL, von Hippel-Lindau tumor suppressor. 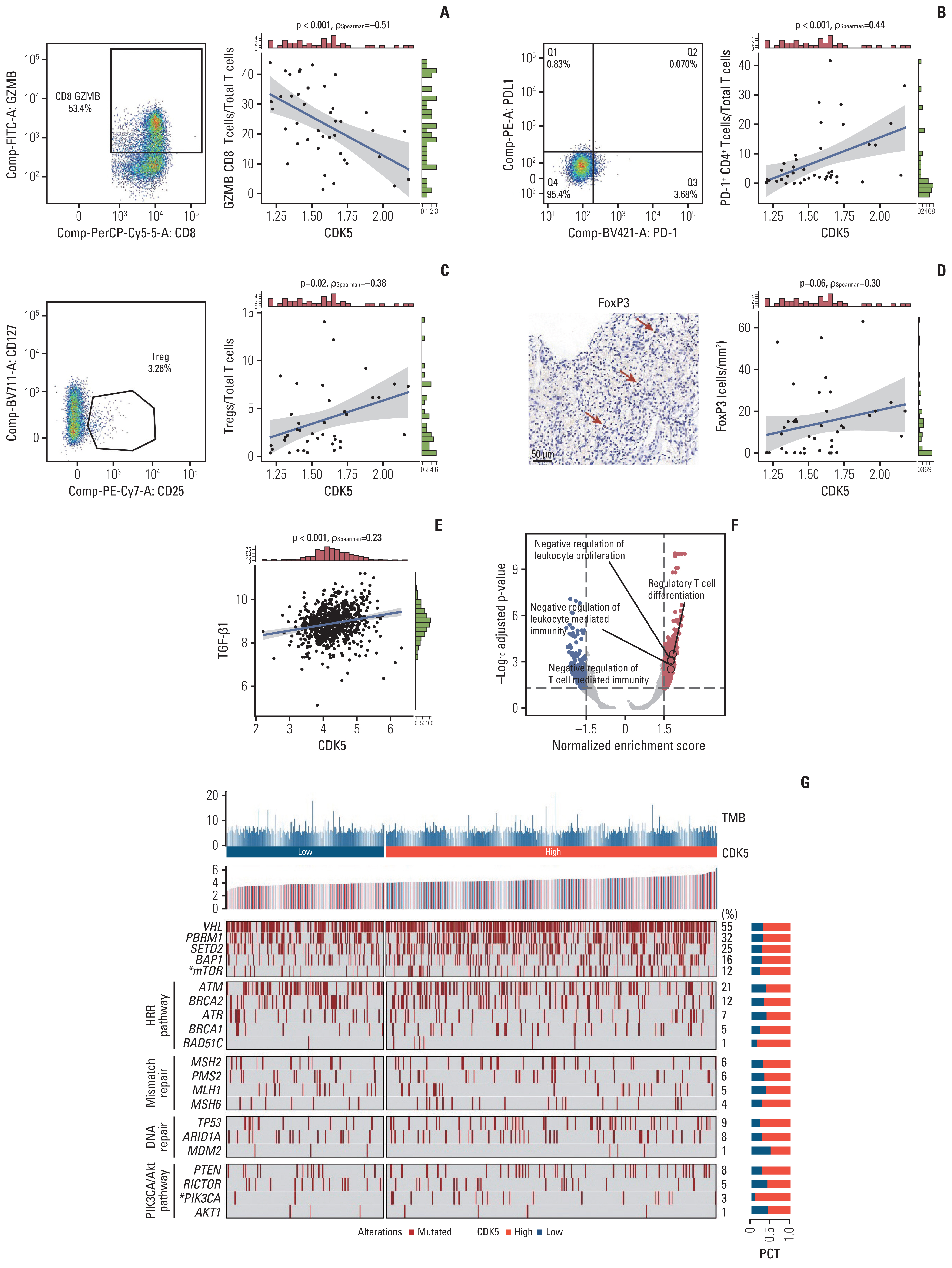
Fig. 5Cyclin-dependent kinase 5 (CDK5) is associated with T cell exhaustion in renal cell carcinoma. (A) Heatmap displaying checkpoints and key transcription factors of T cell exhaustion ranked by CDK5 in the Zhongshan High-risk Localized Renal Cell Carcinoma (ZS-HRRCC) cohort. (B–E) Representative images and quantification of EOMES+ (B), LAG3+ (C), TIGIT+ (D), and TCF1+ cells (E), and their association with CDK5 in ZS-HRRCC cohort by immunohistochemistry. ρ and p-values, Spearman’s rank-order correlation. CTLA4, cytotoxic T lymphocyte antigen 4; EOMES, Eomesodermin; LAG3, lymphocyte activating gene 3; TBX21, T-Box transcription factor 21; TCF1, T cell factor 1; TIGIT, T-cell immunoreceptor with Ig and ITIM domains; TIL, tumor infiltrating lymphocyte; TIM3, T cell immunoglobulin and mucin domain-containing protein 3; TOX, thymocyte selection-associated HMG box. 
Fig. 6An integrated risk score for tyrosine kinase inhibitor (TKI)+immunotherapy (IO) benefit prediction. (A) Variable importance of random forest model parameters, including cyclin-dependent kinase 5 (CDK5), programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), CD8A, CD4, granzyme B (GZMB), and granzyme K (GZMK). (B) The Cox regression model was used to calculate hazard ratio (HR) and 95% confidence interval (CI) of random forest model parameters. HR < 1 indicates better survival with TKI+IO therapy. The cutoff of CDK5 expression was 75%. The cutoffs of the rest of gene expression were median values. HR > 1 indicates better survival with the TKI monotherapy. (C, D) Progression-free survival analysis of advanced renal cell carcinoma with TKI+IO therapy according to risk score in JAVELIN 101 cohort (C) and Zhongshan Metastatic Renal Cell Carcinoma (ZS-MRCC) cohort (D). p-value, Kaplan-Meier analysis, and log-rank test. (E) Kaplan-Meier analysis of advanced RCC with TKI+IO or TKI, in different risk score subgroups. ***p < 0.001. 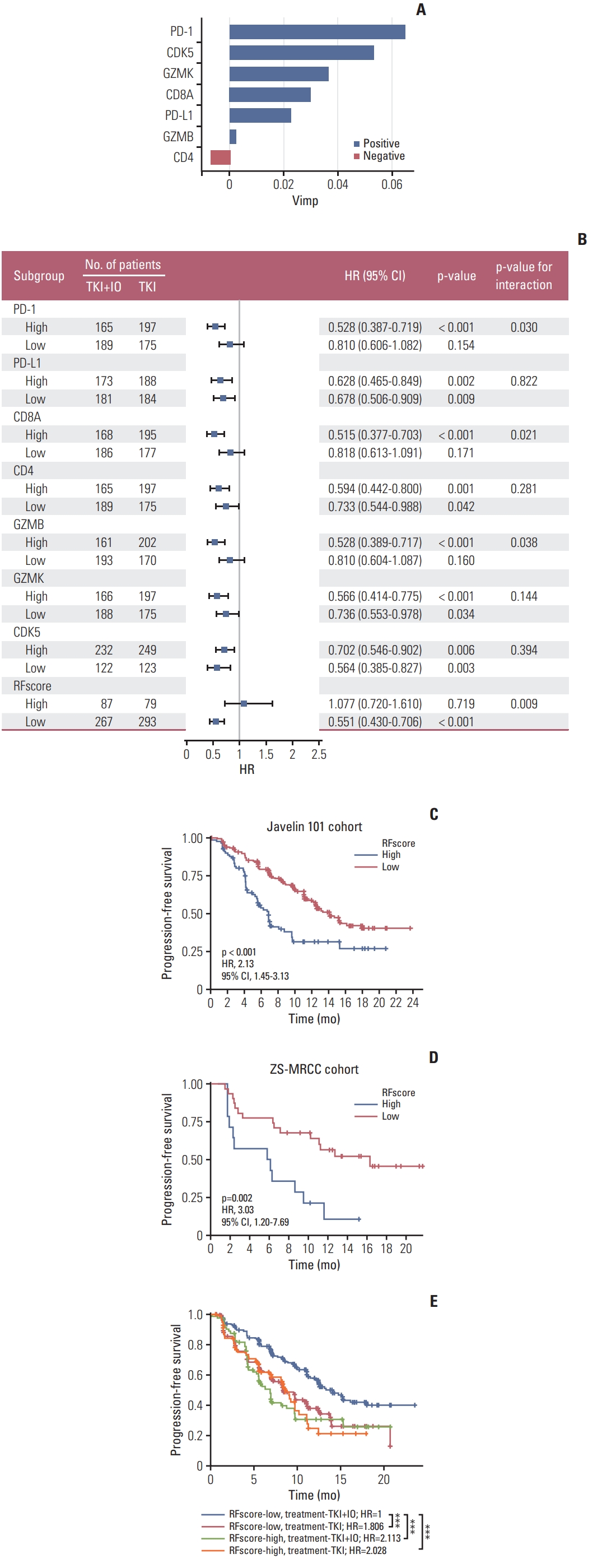
References1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
2. Gill DM, Hahn AW, Hale P, Maughan BL. Overview of current and future first-line systemic therapy for metastatic clear cell renal cell carcinoma. Curr Treat Options Oncol. 2018;19:6.
3. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–15.
4. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27.
5. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90.
6. Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82:399–410.
7. Malumbres M, Harlow E, Hunt T, Hunter T, Lahti JM, Manning G, et al. Cyclin-dependent kinases: a family portrait. Nat Cell Biol. 2009;11:1275–6.
8. Zeng J, Xie S, Liu Y, Shen C, Song X, Zhou GL, et al. CDK5 functions as a tumor promoter in human lung cancer. J Cancer. 2018;9:3950–61.
9. Wang D, Zhou Y, Hua L, Li J, Zhu N, Liu Y. CDK3, CDK5 and CDK8 proteins as prognostic and potential biomarkers in colorectal cancer patients. Int J Gen Med. 2022;15:2233–45.
10. Zhang X, Wang J, Jia Y, Liu T, Wang M, Lv W, et al. CDK5 neutralizes the tumor suppressing effect of BIN1 via mediating phosphorylation of c-MYC at Ser-62 site in NSCLC. Cancer Cell Int. 2019;19:226.
11. Ruiz de Porras V, Bystrup S, Cabrero-de Las Heras S, Musulen E, Palomero L, Alonso MH, et al. Tumor expression of cyclin-dependent kinase 5 (Cdk5) is a prognostic biomarker and predicts outcome of oxaliplatin-treated metastatic colorectal cancer patients. Cancers (Basel). 2019;11:1540.
12. Huang J, Chen P, Liu K, Liu J, Zhou B, Wu R, et al. CDK1/2/5 inhibition overcomes IFNG-mediated adaptive immune resi-stance in pancreatic cancer. Gut. 2021;70:890–9.
13. Dorand RD, Nthale J, Myers JT, Barkauskas DS, Avril S, Chirieleison SM, et al. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science. 2016;353:399–403.
14. Deng H, Tan S, Gao X, Zou C, Xu C, Tu K, et al. Cdk5 knocking out mediated by CRISPR-Cas9 genome editing for PD-L1 attenuation and enhanced antitumor immunity. Acta Pharm Sin B. 2020;10:358–73.
15. De S, Holvey-Bates EG, Mahen K, Willard B, Stark GR. The ubiquitin E3 ligase FBXO22 degrades PD-L1 and sensitizes cancer cells to DNA damage. Proc Natl Acad Sci U S A. 2021;118:e2112674118.
16. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
17. Wang J, Zhang S, Wang Y, Zhu Y, Xu X, Guo J. Alternative complement pathway signature determines immunosuppression and resistance to immunotherapy plus tyrosine kinase inhibitor combinations in renal cell carcinoma. Urol Oncol. 2023;41:51.
18. Xu X, Wang Y, Chen Z, Zhu Y, Wang J, Guo J. Unfavorable immunotherapy plus tyrosine kinase inhibition outcome of metastatic renal cell carcinoma after radical nephrectomy with increased ADAM9 expression. Immunogenetics. 2023;75:133–43.
19. Goldman MJ, Craft B, Hastie M, Repecka K, McDade F, Kamath A, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38:675–8.
20. Wang J, Liu L, Bai Q, Ou C, Xiong Y, Qu Y, et al. Tumor-infiltrating neutrophils predict therapeutic benefit of tyrosine kinase inhibitors in metastatic renal cell carcinoma. Oncoimmunology. 2019;8:e1515611.
21. Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–41.
22. Zhu L, Ding R, Zhang J, Zhang J, Lin Z. Cyclin-dependent kinase 5 acts as a promising biomarker in clear cell renal cell carcinoma. BMC Cancer. 2019;19:698.
23. Liu JL, Wang XY, Huang BX, Zhu F, Zhang RG, Wu G. Expression of CDK5/p35 in resected patients with non-small cell lung cancer: relation to prognosis. Med Oncol. 2011;28:673–8.
24. Liang Q, Li L, Zhang J, Lei Y, Wang L, Liu DX, et al. CDK5 is essential for TGF-beta1-induced epithelial-mesenchymal transition and breast cancer progression. Sci Rep. 2013;3:2932.
25. Chiker S, Pennaneach V, Loew D, Dingli F, Biard D, Cordelieres FP, et al. Cdk5 promotes DNA replication stress checkpoint activation through RPA-32 phosphorylation, and impacts on metastasis free survival in breast cancer patients. Cell Cycle. 2015;14:3066–78.
26. Catania A, Urban S, Yan E, Hao C, Barron G, Allalunis-Turner J. Expression and localization of cyclin-dependent kinase 5 in apoptotic human glioma cells. Neuro Oncol. 2001;3:89–98.
27. Zhang X, Zhong T, Dang Y, Li Z, Li P, Chen G. Aberrant expression of CDK5 infers poor outcomes for nasopharyngeal carcinoma patients. Int J Clin Exp Pathol. 2015;8:8066–74.
28. Futatsugi A, Utreras E, Rudrabhatla P, Jaffe H, Pant HC, Kulkarni AB. Cyclin-dependent kinase 5 regulates E2F transcription factor through phosphorylation of Rb protein in neurons. Cell Cycle. 2012;11:1603–10.
29. Hsu FN, Chen MC, Lin KC, Peng YT, Li PC, Lin E, et al. Cyclin-dependent kinase 5 modulates STAT3 and androgen receptor activation through phosphorylation of Ser(7)(2)(7) on STAT3 in prostate cancer cells. Am J Physiol Endocrinol Metab. 2013;305:E975–86.
30. Lindqvist J, Imanishi SY, Torvaldson E, Malinen M, Remes M, Orn F, et al. Cyclin-dependent kinase 5 acts as a critical determinant of AKT-dependent proliferation and regulates differential gene expression by the androgen receptor in prostate cancer cells. Mol Biol Cell. 2015;26:1971–84.
31. Prince G, Yang TY, Lin H, Chen MC. Mechanistic insight of cyclin-dependent kinase 5 in modulating lung cancer growth. Chin J Physiol. 2019;62:231–40.
32. Lam E, Choi SH, Pareek TK, Kim BG, Letterio JJ. Cyclin-dependent kinase 5 represses Foxp3 gene expression and Treg development through specific phosphorylation of Stat3 at Serine 727. Mol Immunol. 2015;67:317–24.
33. Lam E, Pareek TK, Letterio JJ. Cdk5 controls IL-2 gene expression via repression of the mSin3a-HDAC complex. Cell Cycle. 2015;14:1327–36.
34. Bei Y, Cheng N, Chen T, Shu Y, Yang Y, Yang N, et al. CDK5 inhibition abrogates TNBC stem-cell property and enhances anti-PD-1 therapy. Adv Sci (Weinh). 2020;7:2001417.
35. Gao L, Xia L, Ji W, Zhang Y, Xia W, Lu S. Knockdown of CDK5 down-regulates PD-L1 via the ubiquitination-proteasome pathway and improves antitumor immunity in lung adenocarcinoma. Transl Oncol. 2021;14:101148.
|
|
||||||||||||||||||||||||||||||||||||||||||