AbstractPurposeThe treatment of male breast cancer (MBC) has been extrapolated from female breast cancer (FBC) because of its rarity despite their different clinicopathologic characteristics. We aimed to investigate the distribution of intrinsic subtypes based on immunohistochemistry, their clinical impact, and treatment pattern in clinical practice through a multicenter study in Korea.
Materials and MethodsWe retrospectively analyzed clinical data of 248 MBC patients from 18 institutions across the country from January 1995 to July 2016.
ResultsThe median age of MBC patients was 63 years (range, 25 to 102 years). Among 148 intrinsic subtype classified patients, 61 (41.2%), 44 (29.7%), 29 (19.5%), and 14 (9.5%) were luminal A, luminal B, human epidermal growth factor receptor 2, and triple-negative breast cancer, respectively. Luminal A subtype showed trends for superior survival compared to other subtypes. Most hormone receptor-positive patients (166 patients, 82.6%) received adjuvant endocrine treatment. Five-year completion of adjuvant endocrine treatment was associated with superior disease-free survival (DFS) in patients classified with an intrinsic subtype (hazard ratio [HR], 0.15; 95% confidence interval [CI], 0.04 to 0.49; p=0.002) and in all patients (HR, 0.16; 95% CI, 0.05 to 0.54; p=0.003).
ConclusionDistribution of subtypes of MBC was similar to FBC and luminal type A was most common. Overall survival tended to be improved for luminal A subtype, although there was no statistical significance. Completion of adjuvant endocrine treatment was associated with prolonged DFS in intrinsic subtype classified patients. MBC patients tended to receive less treatment. MBC patients should receive standard treatment according to guidelines as FBC patients.
IntroductionMale breast cancer (MBC) is an orphan disease that accounts for 1% of all breast cancers [1]. In Korea, MBC accounts for approximately 0.36%–0.48% of all breast cancer cases [2,3], which is less frequent than that detected in the United States [1]. Until now, MBC patients have been alienated from various pivotal phase 3 trials due to their rarity in incidence. The treatment guidelines for MBC patients were extrapolated from female breast cancer (FBC) patients, but application of the newest treatment scheme to MBC patients was limited due to lack of evidence based on phase 3 trials that only included FBC patients.
Although the incidence of MBC is rare, it is on the rise [4,5], implying the need for focused studies in this specific population. The clinical and biological characteristics of MBC and FBC are similar in some ways, but there are also many distinct differences between the two disease categories [6]. Risk factors differ from those of FBC; for example, MBC patients tend to harbor BRCA2 mutations rather than BRCA1 mutations compared with FBC [7–10]. MBC tends to be diagnosed at an older age, with a higher incidence of hormone receptor–positive subtype and low incidence of human epidermal growth factor receptor 2 (HER2) compared with FBC [6,11]. Based on immunohistochemical markers or genomic profiling, subtypes of MBC have been previously analyzed in a few studies. Less than 10% of patients were classified as HER2 positive or triple-negative, and most cases were classified as luminal A or luminal B subtypes [11,12]. The distribution pattern and proportion of subtypes were different between MBC and FBC [13,14], and these biological differences may affect the differences in clinical outcomes between the two disease entities. However, the different biological behaviors of MBC were not reflected in the treatment due to the lack of clinical trials focusing on this patient subgroup.
In FBC, treatment is individualized according to the intrinsic subtype, and tailored management, such as anti-HER2 agents and immune-checkpoint inhibitors, has improved survival outcomes. However, as previously mentioned, the treatment of MBC patients is not based on unique biological features and extrapolated from clinical trials of FBC. There are growing efforts to gather data and establish the characteristics of biological features, treatment guidelines, and survival outcomes in MBC [15,16]. However, the studies are focused on the Western population, and the study design is mainly based on a review of previously collected databases that mainly provide basic clinical characteristics, but not sufficient data on pathologic characteristics and in-depth treatment patterns. Previously, we reported the clinical features, treatment patterns, and survival outcomes of MBC patients in seven institutions in Korea [17]. In previously published literature, we have reported that most MBC patients were diagnosed as hormone receptor-positive, and the latest hormonal agents and anti-HER2 treatments were relatively underutilized in these patients. A detailed analysis of the molecular subtype of the enrolled MBC patients was not feasible due to insufficient immunohistochemical surrogate markers, such as the Ki-67 index. Until now, the clinical characteristics and prognosis of Asian MBC patients according to intrinsic subtype have not been well analyzed due to the rarity of its incidence and absence of awareness of this cancer type.
In this study, we conducted a multicenter, retrospective analysis using patients’ baseline demographics and pathologic characteristics, including intrinsic subtype based on immunohistochemical stain, detailed treatment pattern, and clinical outcomes of MBC patients from 18 institutions in Korea during a 21-year period.
Materials and Methods1. PatientsThis retrospective, multicenter study was conducted by the Breast Cancer Division of the Korean Cancer Study Group (KCSG). From January 1995 to July 2016, the medical records of male patients with histologically confirmed breast cancer were retrospectively reviewed. Two-hundred and forty-eight patients primarily diagnosed as MBC were enrolled, and subtypes were able to be analyzed for 148 patients whose data of Ki-67 was available (Fig. 1).
2. Classification of subtype and stageEstrogen receptor (ER) and progesterone receptor (PR) positivity was defined by American Society of Clinical Oncology/College of American Pathologists guideline (ASCO/CAP guideline) [18]. HER2-positive was defined as HER2 immunohistochemistry (IHC) 3+ or HER2 IHC 2+ with HER2 fluorescence or silver in situ hybridization positive based on ASCO/CAP guideline [19,20]. Before introduction of ASCO/CAP guideline, ER, PR, and HER2 positivity was interpreted based on in-house standards in each institution. Intrinsic subtype classification was based on 2015 St. Gallen Consensus Conference recommendations, categorized by IHC results of ER, PR, HER2, and Ki-67 index [21]. Luminal A subtype was defined as ER- and/or PR-positive and HER2-negative with Ki-67 ≤ 20%. Luminal B subtype was defined as ER- and/or PR-positive, HER2-negative with a Ki-67 index > 20%. HER2-enriched subtype was defined as HER2 positive based on IHC of fluorescence in situ hybridization, irrespective of the ER or PR status. Triple-negative breast cancer (TNBC) was defined as ER-, PR-, and HER2-negative. Tumor size and involved number of lymph nodes were collected based on each patient’s medical record. Tumor stage of each patient was redefined based on American Joint Committee on Cancer 7th staging manual [22].
3. Statistical analysisDisease-free survival (DFS) was defined as the period from the time of primary treatment, such as surgical resection, to the date of disease recurrence or death from any cause. Overall survival (OS) was described as the period between the date of the first pathologic diagnosis and death or last follow-up. Continuous variables are presented as median values, and categorical variables are presented as percentages. Continuous variables were compared using the Mann-Whitney U test, while categorical variables were compared using the chi-square test and Fisher exact test. Survival analyses were performed using the Kaplan-Meier method and compared using the log-rank test. Hazard ratios for DFS and OS were estimated using the Cox proportional hazards model with a 95% confidence interval (CI). Two-sided p-values are presented for all analyses with p < 0.05, considered to be statistically significant. R ver. 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
Results1. Patient characteristicsDuring the 21-year follow-up, 248 male patients with breast cancer were enrolled in the study. Baseline patient characteristics are described in Table 1. The median age of the patients was 63 years (range, 25 to 102 years), which was older than that of the Korean FBC patients [23]. Approximately half of the total number of patients were diagnosed before 2010. The number of study registrants tended to increase every 5 years from 1995 to 2016. Most patients (184 patients, 74.2%) complained of a palpable breast mass in the first diagnosis, with a median symptoms’ duration of 6 months (range, 0.2 months to 8 years). Forty percent of patients were diagnosed with stage II breast cancer, while less than 10% were initially diagnosed with stage IV MBC. Ten percent of patients had a family history of cancer (S1 Table), but BRCA mutation status was not assessed in most of them (225 patients, 90.7%). Of the 23 patients analyzed for BRCA mutational status, eight patients were shown to be BRCA mutants.
2. Histopathology of MBCThe histopathology and intrinsic subtype of enrolled patients were analyzed (Fig. 1). Similar to FBC patients, the most common histology of all 248 patients was invasive ductal carcinoma (192 patients, 77.4%). Most patients were diagnosed with hormone receptor–positive MBC (175 patients, 70.6%), with dual expression of the ER and PR (157 patients, 63.3%). Approximately 10% of patients were diagnosed with HER2-positive MBC, and 5% of them were diagnosed with TNBC. Among the 148 patients that underwent subtype classification, most of them were luminal A (61 patients, 41.2%) followed by 44 patients with luminal B disease (29.7%). The incidence of HER2-positive subtype was approximately 20% (29 patients, 19.5%), and TNBC was the least expressed subtype (14 patients, 9.5%) (Table 2).
3. Treatment for early and locally advanced breast cancerUpfront surgery was performed without neoadjuvant chemotherapy in the majority of patients. Most patients underwent modified radical mastectomy (MRM; 165 patients, 72.1%) as surgical treatment. Anthracycline and taxane-based regimen was administered for patients who received neoadjuvant chemotherapy. Adjuvant chemotherapy was delivered to approximately half of the total surgically resected patients, which is a lower rate than that of FBC patients. Anthracycline-based adjuvant chemotherapy was most commonly administered, and approximately one-third of patients received taxane during adjuvant treatment. Few patients received trastuzumab as adjuvant treatment (Table 3). Patients who received adjuvant chemotherapy tended to be younger (median age, 59.5 vs. 70 years; p < 0.001), with good performance status. Adjuvant chemotherapy was mostly administered to patients with a higher tumor stage, histologic tumor grade, and nodal involvement. The adjuvant chemotherapy group tended to receive MRM with axillary node dissection and received adjuvant radiation therapy compared with patients who did not receive adjuvant chemotherapy (S2 Table).
Adjuvant radiation therapy was administered to fewer patients (24% of the 218 operable patients). When analyzed according to the administration of adjuvant radiation therapy, patients with nodal involvement and with higher histologic tumor grade received adjuvant radiation, similar to the adjuvant chemotherapy group. More patients underwent axillary lymph node dissection and neoadjuvant or adjuvant chemotherapy compared with patients who did not receive adjuvant radiation therapy (S3 Table).
Adjuvant endocrine treatment was administered to 166 patients of 200 patients (83%), and approximately 90% of patients received tamoxifen as adjuvant endocrine treatment (S2 Table). Although most hormone receptor–positive MBC patients initiated adjuvant endocrine treatment, approximately 40% of them were able to complete 5 years of adjuvant endocrine treatment (S2 Table).
4. Survival outcomesMedian OS of the total number of treated patients was 60.7 months (range, 1.2 to 230.4 months) and 5-year OS rate was 95.2% (95% CI, 0.921 to 0.984) (Fig. 2A). Older age (> 65 years) (Fig. 2B) and higher stage (Fig. 2C) were significantly associated with inferior survival, with the latter being compared with stage I. Although there were no statistical survival differences according to intrinsic subtype classification based on the Ki-67 index in the analysis, trends for superior OS were observed in luminal subtype compared to non-luminal subtypes (Fig. 3A), and in luminal A subtype when compared to luminal B subtype (Fig. 3B). Among operable hormone receptor-positive MBC patients, completion of adjuvant endocrine treatment was associated with superior DFS and OS compared with patients with incomplete endocrine treatment (median DFS, 13.73 years vs. 76.73 months; p < 0.001 [Fig. 4A]; median OS, 15.98 years vs. 13.88 years; p=0.072 [Fig. 4B]). Contrary to expectations, patients who received adjuvant chemotherapy or radiation therapy showed trends for inferior DFS and OS compared to patients who did not receive adjuvant chemotherapy or radiotherapy (S4 Fig.).
Cox regression analysis was performed for an in-depth analysis of the relationship between clinicopathological variables and survival outcomes (DFS and OS). Completion of adjuvant endocrine treatment was associated with prolonged DFS and OS in patients with MBC (hazard ratio [HR], 0.15; 95% CI, 0.07 to 0.33; p < 0.001; OS: HR, 0.32; 95% CI, 0.09 to 1.11; p=0.073) (Table 4). Older age was also associated with inferior OS (HR, 2.42; 95% CI, 1.13 to 5.16; p=0.023). When adjusted for stage, adjuvant systemic chemotherapy, and radiotherapy, completion of adjuvant endocrine therapy was still associated with prolonged DFS (HR, 0.16; 95% CI, 0.05 to 0.54; p=0.003). Completion of adjuvant endocrine treatment (HR, 0.16; 95% CI, 0.04 to 0.69; p=0.015) and older age (HR, 5.03; 95% CI, 1.13 to 22.4; p=0.034) were associated with OS when adjusted for administration of adjuvant systemic chemotherapy or radiation therapy (Table 4).
In 148 patients who were evaluable for intrinsic subtype classification, 5 years of adjuvant endocrine treatment was still associated with superior DFS (HR, 0.22; 95% CI, 0.07 to 0.63; p=0.004). When adjusted for stage, subtype, adjuvant chemotherapy, and radiotherapy, completion of adjuvant endocrine treatment remained to be associated with prolonged DFS in the group of patients that were classified according to the intrinsic subtype (HR, 0.15; 95% CI, 0.04 to 0.49; p=0.002) (Table 5).
5. Palliative treatment for recurrent, metastatic MBCDuring a median follow-up of 59.6 months, 51 patients (22.2%) experienced disease recurrence. Nineteen patients (7.7%) were diagnosed with de novo stage IV MBC, and four patients (1.6%) were diagnosed with locally advanced disease at initial presentation. Among the 60 patients who were treated for recurrent or metastatic MBC, 43 (71.7%) received palliative treatment. The treatment patterns were analyzed based on the decade period. Although there were a small number of recurrent, metastatic HER2-positive patients (7 patients) in our study, anti-HER2 treatment, such as trastuzumab, pertuzumab, lapatinib, and trastuzumab emtansine were underutilized, and none of the patients received trastuzumab before 2010. During that period, patients received a combination of chemotherapy with taxane and anthracycline. After 2010, capecitabine was more frequently used in earlier settings, while monotherapy was utilized more often (S5 Table).
Among the 56 patients who were hormone receptor-positive, 35 (62.5%) received palliative endocrine treatment. Tamoxifen was the most frequently used endocrine treatment in MBC patients as a first-line treatment. Aromatase inhibitors were administered as first-line or above in patients, but few patients received concurrent gonadotropin-releasing hormone (GnRH) agonists during treatment. Although BOLERO-2 [24] enrolled postmenopausal women, three hormone receptor-positive MBC patients in our analysis used exemestane with everolimus for their treatment. However, GnRH agonists were not administered concurrently during treatment. None of the patients were treated with fulvestrant (S6 Table).
DiscussionIn this study, we intensively reviewed the medical records of each enrolled patient from multiple tertiary medical centers who were qualified for breast cancer treatment. Based on the data collection over more than 20 years, we analyzed the clinicopathological characteristics, including intrinsic subtype, pattern of treatment during adjuvant and palliative setting, and survival outcome.
The median age of the total patient population was 63 years, similar to previous Korean Central Cancer Registry data and the Western registry data [3,25,26]. The median age of the enrolled patients was 10 years older than that of the FBC patients in Korea [23], suggesting possible characteristics of aging-associated disease of MBC compared with FBC [6]. Previous reports have proposed that the late onset of MBC may be due to delayed recognition and evaluation of symptoms and signs of breast cancer [27]. However, recent studies based on genomic profiling have suggested that the biological characteristics of MBC may differ from those of FBC [12,28]. Differences in genomic profiles may have affected the distinct clinicopathological characteristics between MBC and FBC. In our analysis, the proportion of hormone receptor–positive patients is higher and the incidence of TNBC is lower than that of FBC as previously reported [11]. Among intrinsic subtype classified patients, HER2-positive patients were higher than previously reported literature [11]. This discrepancy may be due to selection bias, considering that a certain portion of old archival tissue was not tested for HER2 and the Ki-67 index was not fully analyzed in all patients.
Considering that most patients with early-stage MBC are diagnosed as hormone receptor–positive, adjuvant endocrine treatment may have an important role in extending survival. In our study more than 80% of hormone receptor positive patients started adjuvant endocrine treatment but less than half of patients have completed 5 years of treatment. Considering the side effects of adjuvant endocrine treatment, long-term compliance has always been an issue in the treatment of MBC [29]. Previous studies have demonstrated that the use of adjuvant endocrine treatment is associated with improved survival [25], but treatment compliance has not been analyzed due to the limited assessment of medical records of each patient [26]. Completion of adjuvant endocrine treatment was significantly associated with prolonged DFS and OS, and still showed survival benefit when adjusted for other clinicopathological factors, such as tumor stage, history of adjuvant chemotherapy, or radiotherapy. Completion of adjuvant endocrine treatment was also associated with superior DFS in intrinsic subtype classified patients. Our analysis suggests that adherence to adjuvant endocrine treatment is the most important factor for prolonged survival in early-stage MBC, and adequate supportive care is also warranted to improve compliance with adjuvant endocrine treatment.
In MBC patients, neoadjuvant chemotherapy, adjuvant chemotherapy and adjuvant radiation treatment were not performed as often as in FBC. This result is in agreement with the Korea Central Cancer Registry data [3]. Compared with US data on MBC, a similar proportion of patients was treated with adjuvant chemotherapy, but fewer patients received adjuvant radiation therapy in our analysis [25,26]. No prospective randomized trials have evaluated the role of adjuvant chemotherapy or radiotherapy in MBC. Based on observational studies, adjuvant treatment is recommended, especially for node-positive disease [6]. However, MBC has been neglected and underestimated due to its rarity and this has influenced relative undertreatment in patient population. In our analysis, relatively young patients with good performance status diagnosed with more advanced stage received adjuvant chemotherapy or radiotherapy (S2 and S3 Tables). This limited assessment to adjuvant treatment might have influenced unexpected trends for inferior DFS or OS in patients who received adjuvant treatment.
Excluding MBC patients in various pivotal phase 3 trials has resulted in a relative lack of evidence when establishing treatment guidelines in various countries [6,26]. Therefore, the application of the latest treatment for MBC is frequently delayed due to regulatory issues in various countries. We showed that anti-HER2 agents were underutilized in HER2-positive MBC patients, and hormone receptor–positive patients had also limited assessment for fulvestrant and everolimus. Older drugs such as tamoxifen, taxane, anthracycline, and 5-fluorouracil were preferentially administered to patients in this study, while relatively few patients had the chance to receive novel anti-HER2 agents, such as trastuzumab, lapatinib, and trastuzumab emtansine. Considering most MBC patients were diagnosed as hormone receptor positive, treatment pattern in palliative endocrine regimen is quite important. Hormone positive MBC patients who were treated with aromatase inhibitor, exemestane with everolimus did not receive GnRH agonist, which might have reduced the treatment efficacy.
In our analysis, the 5-year OS was estimated to be over 90%, which is superior to previously published literature ranging from 77% to 87% [3,17,25,26]. The survival rate of MBC patients was comparable with that of patients with FBC [23]. There are conflicting reports about the survival of MBC compared with FBC [30,31]. Former literature has reported inferior OS of MBC may be due to the initial advanced stage, underlying comorbidity, and older age at diagnosis when compared with women [6]. However, there are incompatible report that MBC showed a superior survival rate compared with those with FBC when adjusted for stage, treatment, and demographic features [27]. Compared to other studies, higher proportion of adjuvant endocrine treated patient population might have influenced the better survival outcome in our study [25,26]. Considering completion of adjuvant endocrine treatment is also important in extending survival, this treatment pattern might have also influenced survival outcomes comparable with those of FBC.
The strength of this study is the long-term follow-up data that were collected in multiple institutions, spanning more than 20 years with detailed medical records of enrolled patients, although it was based on retrospective analysis. To the best of our knowledge, this is the first report analyzing the intrinsic subtype based on IHC for the Asian patients with MBC. Intrinsic subtype is originally defined after gene expression profiling, but the classification of intrinsic subtype was based only on IHC performed at each institution without central review. Gene expression profiling was not conducted due to lack of archival tumor tissues and poor quality of long-term stored formalin fixed paraffin embedded tissues. Although investigators have reviewed the medical records of each enrolled patient, pathological characteristics, such as Ki-67 index, were omitted or not analyzed in some patients that were diagnosed in the nineties or the early 2000s. Therefore, approximately half of the patients could not be classified for subtype analysis. Additionally, most patients did not undergo a germline BRCA1/BRCA2 mutation analysis, and their family cancer history or presence of secondary malignancies were omitted. Although BRCA mutation is a well-known contributing factor for the development of MBC, low recognition of clinicopathological characteristics of MBC and absence of national insurance coverage for laboratory analysis have influenced the lower detection rate of BRCA mutations in clinical settings. Nowadays, as there is a better understanding of the clinicopathological features of MBC, there is a growing rate of analysis of BRCA status and other genomic tests, such as Oncotype Dx and MammaPrint [32,33]. Analysis of the relationship between molecular profiles and germline mutations, such as BRCA mutations, with clinical characteristics and prognosis of MBC patients, may be warranted for future in-depth study.
To conclude, this was the first study analyzing subtypes of Korean MBC patients based on a multicenter study. The luminal A subtype was the most common, and completion of adjuvant endocrine treatment in patients that were classified based on the subtype was associated with prolonged DFS. Completion of adjuvant endocrine treatment was also the most important factor for prolonged DFS and OS in hormone receptor-positive MBC. Known prognostic factors were adjusted during analysis of relationship between prognostic factors and DFS or OS. Although statistically non-significant due to small number of patients, intrinsic subtype showed trend with improved survival for luminal A subtype. The incidence of MBC patients has increased recently, and appropriate treatment is this patient group is warranted. To improve survival of MBC patients, they should receive standard treatment according to guidelines as FBC patients.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the institutional review board of each participating institution. The requirement for informed consent was waived because of the retrospective nature of this study. Author Contributions Conceived and designed the analysis: Woo IS. Collected the data: Lee J, Lee KS, Sim SH, Chae H, Sohn J, Kim GM, Lee KH, Kang SH, Jung KH, Jeong JH, Byun JH, Koh SJ, Lee KE, Lim S, Kim HJ, Park HS, Lee GJ, Hong S, Baek SK, Lee SI, Choi MY, Woo IS. Contributed data or analysis tools: Lee J, Lee KS, Sim SH, Chae H, Sohn J, Kim GM, Lee KH, Kang SH, Jung KH, Jeong JH, Byun JH, Koh SJ, Lee KE, Lim S, Kim HJ, Won HS, Park HS, Lee GJ, Hong S, Baek SK, Lee SI, Choi MY, Woo IS. Performed the analysis: Lee J. Wrote the paper: Lee J, Woo IS. Fig. 2Survival outcomes in treated patients: (A) in all treated patients, (B) overall survival according to age, and (C) overall survival according to stage. CI, confidence interval; HR, hazard ratio; mOS, median overall survival; NA, not available; OS, overall survival. 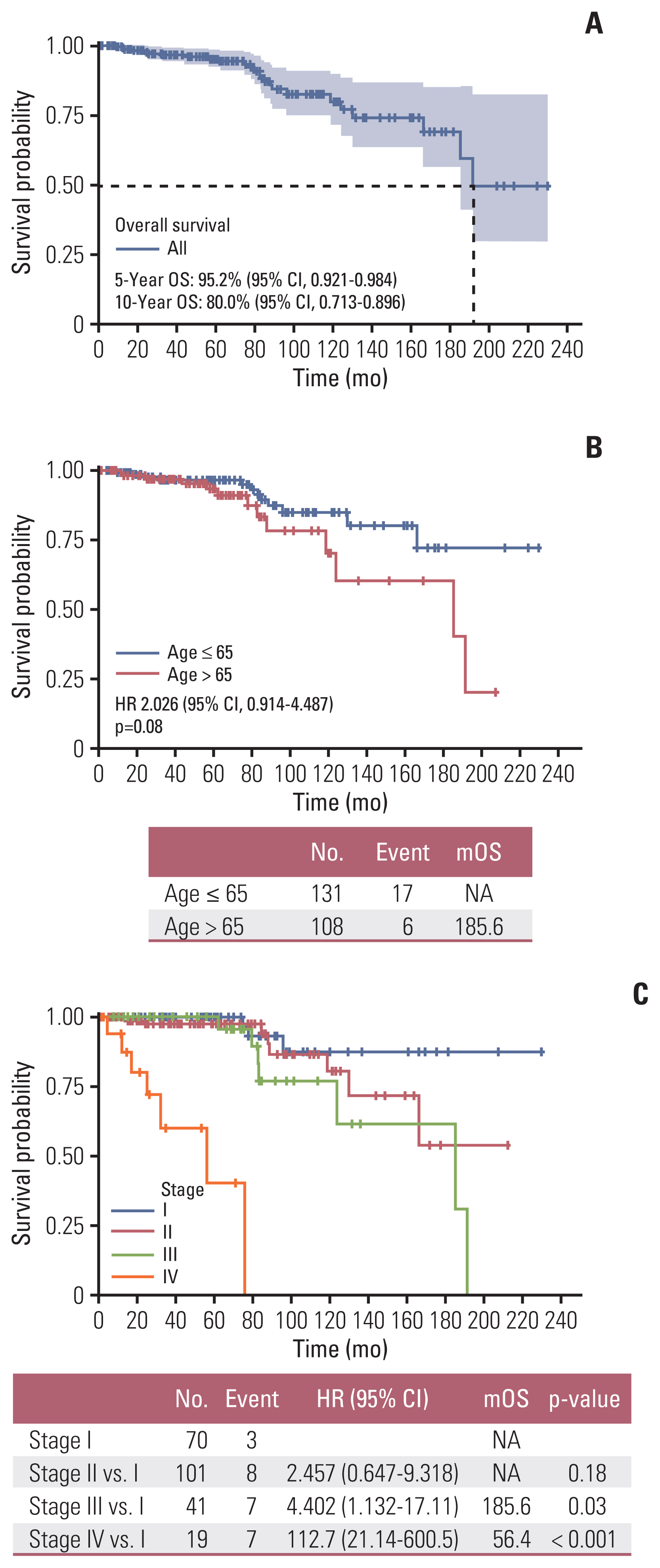
Fig. 3Survival outcomes according to subtypes. (A) Comparison of OS between luminal and non-luminal subtypes. (B) Comparison of OS between luminal A and other subtypes (n=148). CI, confidence interval; HR, hazard ratio; OS, overall survival. 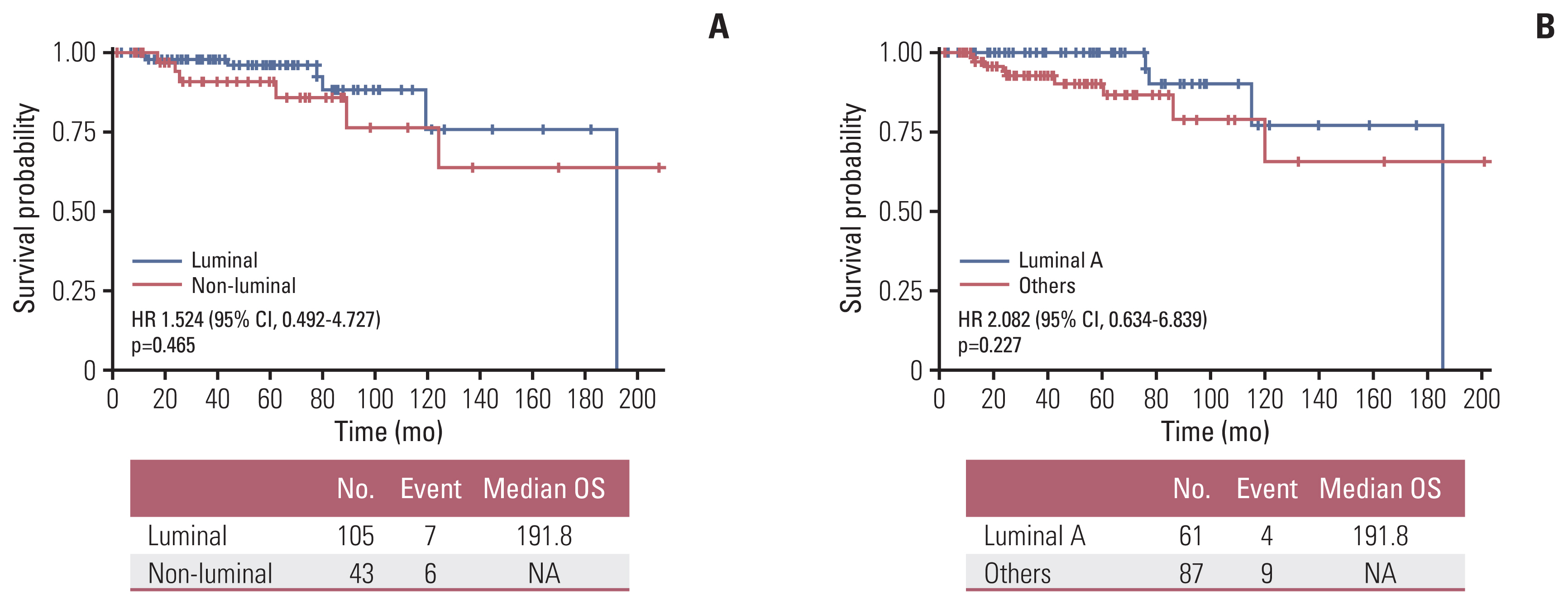
Fig. 4Survival outcomes according to completion of adjuvant endocrine treatment in hormone receptor–positive subgroup. (A) DFS according to completion of adjuvant endocrine treatment. (B) OS according to completion of adjuvant endocrine treatment. CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; NA, not available; OS, overall survival. 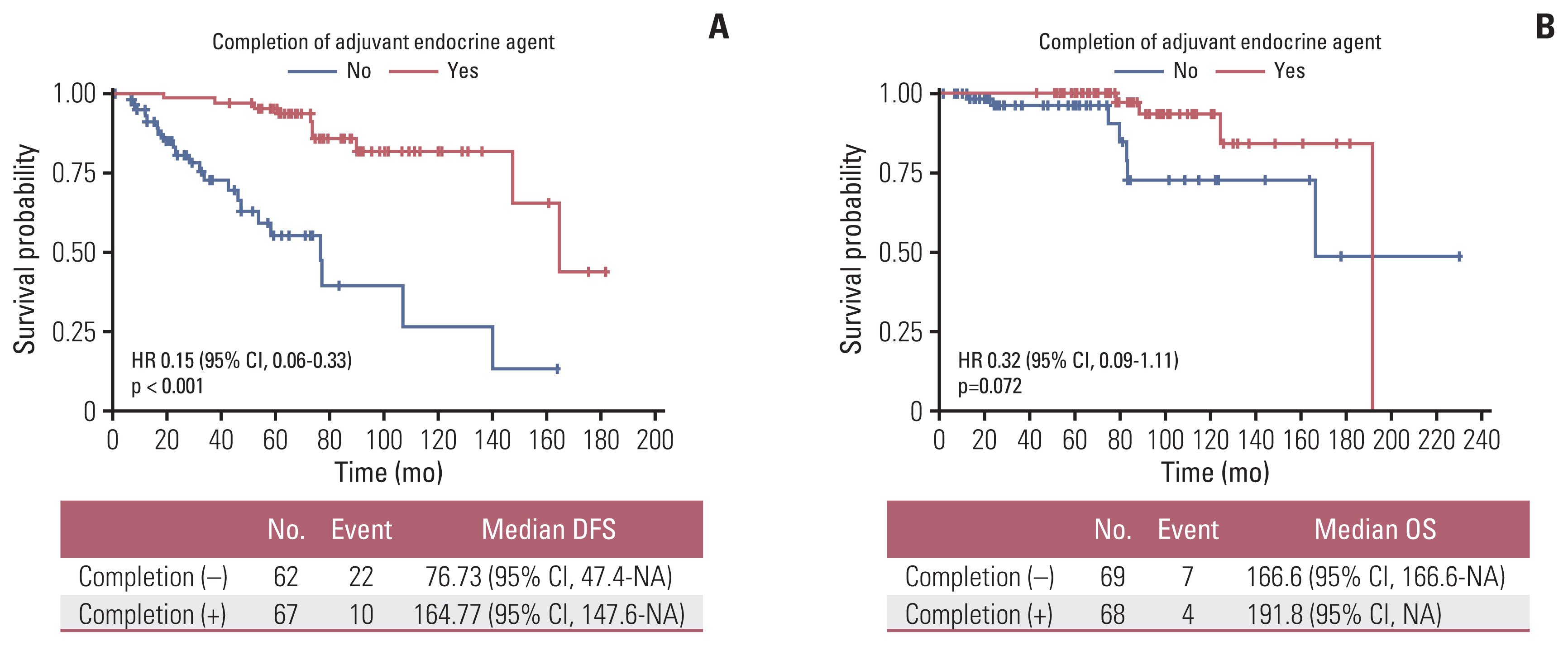
Table 1Baseline patient characteristics
Table 2Histopathologic characteristics of patient population Table 3Treatment patterns in operable patients AC, adriamycin-cyclophosphamide; ALND, axillary lymph node dissection; CMF, cyclophosphamide-methotrexate-5-fluorouracil; DA, docetaxel-adriamycin; EC, epirubicin-cyclophosphamide; FAC, 5-fluorouracil-adriamycin-cyclophosphamide; FEC, 5-fluorouracil-epirubicin-cyclophosphamide; SLNB, sentinel lymph node biopsy; TAC, docetaxel-adriamycin-cyclophosphamide; TC, docetaxel-cyclophosphamide. Table 4Univariate and multivariate Cox regression analysis for DFS and OS in total patient population Table 5Univariate and multivariate Cox regression analysis for DFS and OS in intrinsic subtype classified patients References2. Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021;53:301–15.
3. Lee EG, Jung SY, Lim MC, Lim J, Kang HS, Lee S, et al. Comparing the characteristics and outcomes of male and female breast cancer patients in Korea: Korea Central Cancer Registry. Cancer Res Treat. 2020;52:739–46.
4. Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975–2015. Bethesda, MD: National Cancer Institute; 2018.
5. Abdelwahab Yousef AJ. Male breast cancer: epidemiology and risk factors. Semin Oncol. 2017;44:267–72.
7. Basham VM, Lipscombe JM, Ward JM, Gayther SA, Ponder BA, Easton DF, et al. BRCA1 and BRCA2 mutations in a population-based study of male breast cancer. Breast Cancer Res. 2002;4:R2.
8. Ding YC, Steele L, Kuan CJ, Greilac S, Neuhausen SL. Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res Treat. 2011;126:771–8.
9. Friedman LS, Gayther SA, Kurosaki T, Gordon D, Noble B, Casey G, et al. Mutation analysis of BRCA1 and BRCA2 in a male breast cancer population. Am J Hum Genet. 1997;60:313–9.
10. Ottini L, Masala G, D’Amico C, Mancini B, Saieva C, Aceto G, et al. BRCA1 and BRCA2 mutation status and tumor characteristics in male breast cancer: a population-based study in Italy. Cancer Res. 2003;63:342–7.
11. Cardoso F, Bartlett JM, Slaets L, van Deurzen CH, van Leeuwen-Stok E, Porter P, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018;29:405–17.
12. Piscuoglio S, Ng CK, Murray MP, Guerini-Rocco E, Martelotto LG, Geyer FC, et al. The genomic landscape of male breast cancers. Clin Cancer Res. 2016;22:4045–56.
13. Callari M, Cappelletti V, De Cecco L, Musella V, Miodini P, Veneroni S, et al. Gene expression analysis reveals a different transcriptomic landscape in female and male breast cancer. Breast Cancer Res Treat. 2011;127:601–10.
14. Shaaban AM, Ball GR, Brannan RA, Cserni G, Di Benedetto A, Dent J, et al. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res Treat. 2012;133:949–58.
15. Hassett MJ, Somerfield MR, Baker ER, Cardoso F, Kansal KJ, Kwait DC, et al. Management of male breast cancer: ASCO guideline. J Clin Oncol. 2020;38:1849–63.
16. Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28:2114–22.
17. Hong JH, Ha KS, Jung YH, Won HS, An HJ, Lee GJ, et al. Clinical features of male breast cancer: experiences from seven institutions over 20 years. Cancer Res Treat. 2016;48:1389–98.
18. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134:e48–72.
19. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43.
20. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
21. Gnant M, Thomssen C, Harbeck N. St. Gallen/Vienna 2015: a brief summary of the consensus discussion. Breast Care (Basel). 2015;10:124–30.
22. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
23. Korean Breast Cancer Society. Breast Cancer Facts and Figures 2020. Seoul: Korean Breast Cancer Society; 2020.
24. Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9.
25. Sarmiento S, McColl M, Musavi L, Gani F, Canner JK, Jacobs L, et al. Male breast cancer: a closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Res Treat. 2020;180:471–9.
26. Yadav S, Karam D, Bin Riaz I, Xie H, Durani U, Duma N, et al. Male breast cancer in the United States: treatment patterns and prognostic factors in the 21st century. Cancer. 2020;126:26–36.
27. Miao H, Verkooijen HM, Chia KS, Bouchardy C, Pukkala E, Laronningen S, et al. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011;29:4381–6.
28. Johansson I, Ringner M, Hedenfalk I. The landscape of candidate driver genes differs between male and female breast cancer. PLoS One. 2013;8:e78299.
29. Oke O, Niu J, Chavez-MacGregor M, Zhao H, Giordano SH. Adjuvant tamoxifen adherence in men with early-stage breast cancer. Cancer. 2022;128:59–64.
30. Gnerlich JL, Deshpande AD, Jeffe DB, Seelam S, Kimbuende E, Margenthaler JA. Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol. 2011;18:1837–44.
31. Marchal F, Salou M, Marchal C, Lesur A, Desandes E. Men with breast cancer have same disease-specific and event-free survival as women. Ann Surg Oncol. 2009;16:972–8.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||