AbstractPurposeWe evaluated study outcomes in patients enrolled in Asian regions in the phase III EMBRACA trial of talazoparib vs. chemotherapy.
Materials and MethodsPatients with human epidermal growth factor receptor 2–negative germline BRCA1/2-mutated advanced breast cancer who received prior chemotherapy were randomized 2:1 to talazoparib 1 mg/day or chemotherapy (physician’s choice). Primary endpoint was progression-free survival (PFS) per independent central review in the intent-to-treat (ITT) population. This post-hoc analysis evaluated efficacy/safety endpoints in the ITT population of patients enrolled in Asian regions.
ResultsThirty-three patients were enrolled at Asian sites (talazoparib, n=23; chemotherapy, n=10). Baseline characteristics were generally comparable with the overall EMBRACA population. In Asian patients, median PFS was 9.0 months (95% confidence interval [CI], 3.0 to 15.2) for talazoparib and 7.1 months (95% CI, 1.2 to not reached) for chemotherapy (hazard ratio [HR], 0.74 [95% CI, 0.22 to 2.44]). Objective response rate was numerically higher for talazoparib vs. chemotherapy (62.5% [95% CI, 35.4 to 84.8] vs. 25.0% [95% CI, 3.2 to 65.1]). Median overall survival was 20.7 months (95% CI, 9.4 to 40.1) versus 21.2 months (95% CI, 2.7 to 35.0) (HR, 1.41 [95% CI, 0.49 to 4.05]). In Asian patients, fewer grade 3/4 adverse events (AEs), serious AEs (SAEs), grade 3/4 SAEs, and AEs resulting in dose reduction/discontinuation occurred with talazoparib than chemotherapy; for talazoparib, the frequency of these events was lower in Asian patients versus overall EMBRACA population.
IntroductionMutations in germline breast cancer susceptibility genes 1 or 2 (gBRCA1/2) result in a compromised ability to repair DNA double-strand breaks by homologous recombination, rendering cancer cells highly sensitive to the poly(ADP- ribose) polymerase (PARP) enzyme pathway for DNA repair [1–4]. Consequently, tumors harboring gBRCA1/2 mutations are highly sensitive to targeted treatment with PARP inhibitors, which cause cell death due to accumulation of unrepaired DNA damage [1,3].
Talazoparib is a second generation, orally available PARP inhibitor that inhibits catalytic activity and traps PARP on DNA, disrupting DNA damage repair and causing death in cells with deleterious mutations in BRCA1/2 [4]. Talazoparib has been shown to have equivalent catalytic activity compared with other PARP inhibitors, but is approximately 100-fold more potent at trapping PARP–DNA complexes, a mechanism that appears to be more cytotoxic than catalytic inhibition of PARP alone [5–7]. In the phase III EMBRACA trial (NCT01945775), involving 431 patients with human epidermal growth factor 2 (HER2)–negative locally advanced/metastatic breast cancer and a gBRCA1/2 mutation, treatment with talazoparib 1 mg/day significantly improved progression-free survival (PFS) compared with physician’s choice of single-agent chemotherapy treatment (median, 8.6 months vs. 5.6 months; hazard ratio [HR], 0.54; 95% confidence interval [CI], 0.41 to 0.71; p < 0.001) [8]. Talazoparib showed a manageable safety profile, and significantly improved patient-reported outcomes compared with chemotherapy. Based on these results, talazoparib has been approved in the United States, European Union, and other countries for the treatment of patients with gBRCA1/2 mutations who have HER2-negative locally advanced/metastatic breast cancer and a gBRCA1/2 mutation [9,10].
The prevalence of BRCA1 and BRCA2 mutations in unselected Asian populations with breast cancer is reported to be similar to that observed in most Western patient populations, ranging from approximately 1.5% to 2.0% and 1.5% to 4.0% for each gene, respectively [11,12]. However, the apparent higher prevalence of BRCA2 mutations compared with BRCA1 mutations is a distinct feature in East Asian patients [13–15]. The spectrum of gBRCA1/2 variants also varies across different ethnic groups; importantly, there is a relatively high frequency of variants of uncertain significance in Asian patients [15]. To investigate this further, a recent study analyzed over 78,000 samples from cancer patients and 40,000 samples from non-cancer patients in Indian, Chinese, Korean, and Japanese populations, and found that over half of the BRCA variants identified were Asian specific [16]. Currently, there is also a paucity of data regarding the use of PARP inhibitors, including talazoparib, in Asian patients, owing to the predominance of Western patients in some breast cancer clinical trials [17–19]. Subgroup analysis from the EMBRACA study showed prolonged PFS with talazoparib versus chemotherapy in all clinically relevant patient subgroups, including non-White populations [17]. However, there are reports of epidemiologic and clinicopathologic differences between Asian and Western women with breast cancer, such as a higher incidence at younger age among Asian women and age-specific disparities in hormone receptor positivity between Asian and Western populations [20]. Nonetheless, the PFS improvement with talazoparib compared with chemotherapy was consistent regardless of age or hormone receptor status within the full intent-to-treat (ITT) population [17]. Despite these findings, it is important to characterize the efficacy and safety of talazoparib specifically in patients from within Asia, due to additional differences (e.g., genetic background, socio-economic profile, culture) observed between patients from Asian and Western countries that may have clinical implications for breast cancer treatment [15,21]. This subgroup analysis reports efficacy and safety outcomes from 33 patients enrolled at Asian sites (Korea and Taiwan) in the EMBRACA trial.
Materials and Methods1. Study design and patientsEMBRACA was an open-label, randomized, multicenter, phase III trial comparing the efficacy and safety of talazoparib with chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine) in patients with HER2-negative advanced breast cancer and a gBRCA1/2 mutation, who had received prior chemotherapy for metastatic disease.
The study design has been published previously [8,22]. Briefly, eligible patients were at least 18 years old, with HER2-negative locally advanced/metastatic breast cancer with documentation of a deleterious, suspected deleterious, or pathogenic gBRCA1/2 mutation by central testing. Patients must have received no more than three previous cytotoxic regimens for advanced breast cancer, and have received previous treatment with a taxane and/or anthracycline, unless contraindicated. Previous neoadjuvant or adjuvant platinum-based therapy was allowed, providing patients had not relapsed within 6 months of the last dose. Exclusion criteria included disease progression on platinum-based chemotherapy for locally advanced/metastatic breast cancer; cytotoxic chemotherapy, radiation therapy, antihormonal therapy, or other targeted anticancer therapy within 14 days before randomization; and untreated central nervous system (CNS) metastases (patients with stable, adequately treated CNS metastases were allowed).
Patients were randomized in a 2:1 ratio to talazoparib or single-agent chemotherapy, stratified by the number of prior cytotoxic chemotherapy regimens (0 vs. 1, 2, or 3), triple-negative breast cancer status (hormone receptor-negative and HER2-negative) based on the most recent biopsy (yes vs. no), and history of CNS metastases (yes vs. no). Talazoparib 1 mg was administered orally once daily. The chemotherapy group received protocol-specified chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine) in continuous 21-day cycles, with the choice of chemotherapy determined before randomization. Laboratory values were monitored every 3 weeks and patients were required to have adequate counts (hemaglobin ≥ 8.0 g/dL, absolute neutrophil count ≥ 1,500/mm3, platelets ≥ 50,000/mm3) to continue treatment with talazoparib; further details can be found in Litton et al. [8]. Treatment continued until radiographic disease progression, unacceptable toxicity, withdrawal of consent, or until the physician decided to end treatment.
The current exploratory analysis comprises patients who were enrolled at sites in Korea and Taiwan (referred to as the Asian subgroup or Asian ITT/safety population).
2. Outcomes and assessmentsThe primary endpoint was radiologic PFS, determined by blinded independent central review per Response Evaluation Criteria in Solid Tumors ver. 1.1 (RECIST v1.1). Secondary efficacy endpoints included objective response rate (ORR) per investigator review (RECIST v1.1) in patients with measurable disease, and overall survival (OS). Duration of objective response was an exploratory endpoint. Tumor assessments were performed every 6 weeks until week 30, and every 9 weeks thereafter until progressive disease per central review or the start of a new antineoplastic therapy. After treatment discontinuation, patients were followed every 12 weeks for survival status and use of subsequent anticancer treatment. Safety was assessed based on adverse events (AEs), graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, ver. 4.03, and clinically relevant changes in laboratory values.
3. Statistical analysesWith the exception of OS, efficacy and safety data are based on the first data cutoff (September 15, 2017); final OS data are based on a data cutoff date of September 30, 2019.
Details of the statistical analyses have been described previously [8]. Median PFS and OS were estimated using Kaplan-Meier methodology, with stratified log-rank test and HRs estimated via a stratified Cox regression model used to compare treatment groups. Efficacy analyses were performed in the ITT population, which included all randomized patients. Exposure and safety were evaluated in the safety population, comprising all patients who received at least one dose of study drug.
Results1. Patients and treatmentBetween October 2013 and April 2017, 33 patients were enrolled at sites in Asia and were randomly assigned to talazoparib (n=23) or chemotherapy (n=10) (Fig. 1). At data cutoff (September 15, 2017), 20 patients (87%) in the talazoparib arm and all patients in the chemotherapy arm had discontinued treatment, predominantly due to progressive disease.
Demographic and baseline disease characteristics were generally similar between the Asian ITT population and the overall EMBRACA ITT population (Table 1). Exceptions included patients in the Asian subgroup tending to be slightly younger versus the overall population in both the talazoparib and chemotherapy arms, and more chemotherapy-treated patients in the Asian subgroup had BRCA2 mutations than in the overall chemotherapy-treated population. In the chemotherapy group, there was also a slightly lower proportion of patients with visceral disease in the Asian subgroup compared with the overall population.
2. EfficacyIn total, 24 PFS events by blinded central review occurred among patients enrolled in Asia (18/23 [78%] in the talazoparib arm and 6/10 [60%] in the chemotherapy arm) (Fig. 2A). Median PFS by blinded central review in the Asian ITT population was 9.0 months (95% CI, 3.0 to 15.2) for talazoparib and 7.1 months (95% CI, 1.2 to not reached) for chemotherapy (HR, 0.74 [95% CI, 0.22 to 2.44]). PFS in the overall ITT population is shown for comparison in Fig. 2B. Median PFS per investigator assessment in the Asian ITT population was 7.0 months (95% CI, 4.0 to 13.0) for talazoparib and 4.8 months (95% CI, 1.2 to 8.8) for chemotherapy (HR, 0.70 [95% CI, 0.23 to 2.08]) (Fig. 2C). PFS results for the overall ITT population based on investigator assessment are shown in Fig. 2D.
For patients in the Asian ITT population with measurable disease, the ORR was numerically higher in the talazoparib arm than in the chemotherapy arm (62.5% [95% CI, 35.4 to 84.8] vs. 25.0% [95% CI, 3.2 to 65.1]; odds ratio, 1.88 [95% CI, 0.07 to 117.85]) (Table 2). Median duration of objective response was 9.5 months (95% CI, 1.0 to 14.4) for talazoparib and 5.2 months (95% CI, 2.8 to 7.6) for chemotherapy. ORR results for the overall ITT population are also shown in Table 2.
At data cutoff for OS (September 30, 2019), 25 patients in the Asian ITT population had died (17/23 [74%] in the talazoparib arm and 8/10 [80%] in the chemotherapy arm). Median OS in the Asian ITT population was 20.7 months (95% CI, 9.4 to 40.1) for talazoparib and 21.2 months (95% CI, 2.7 to 35.0) for chemotherapy (HR, 1.41 [95% CI, 0.49 to 4.05]) (Table 3). OS results for the overall ITT population are also shown in Table 3.
3. Exposure and safetyAll patients in the Asian subgroup (n=33) received at least one dose of study drug and were included in the safety population. Patients in the talazoparib and chemotherapy arms received a median (range) of 5.7 months (1.9 to 23.5) and 4.9 months (1.2 to 13.1) of treatment, respectively (Table 4). The median relative dose intensity of talazoparib was higher in the Asian subgroup versus the overall safety population (99.7% [n=23] vs. 87.2% [n=286]). The median relative dose intensity of chemotherapy was similar between the Asian subgroup and the overall safety population (eribulin, 93.8% [n=3] vs. 96.4% [n=50]; vinorelbine, 65.0% [n=3] vs. 64.3% [n=9]; no patients received gemcitabine), with the exception of a higher relative dose intensity of capecitabine in Asian patients (Asian subgroup, 99.6% [n=4]; overall safety population, 87.9% [n=55]) (Table 4). In the talazoparib arm, five patients (21.7%) in the Asian safety population and 149 patients (52.1%) in the overall safety population had at least one dose reduction due to AEs (Table 4). Most patients in the Asian safety population had only one dose reduction (n=3, 13%); only one patient each had two or three dose reductions.
An overall summary of treatment-emergent AEs (TEAEs) is shown in Table 5. Among patients in the Asian safety population, fewer grade 3/4 TEAEs, serious AEs (SAEs), grade 3/4 SAEs, and TEAEs resulting in dose modification (comprising both temporary interruptions and dose reductions) occurred in the talazoparib arm than in the chemotherapy arm. The frequency of these events was also lower in the Asian subgroup than in the overall safety population in the talazoparib arm but was similar across both populations in the chemotherapy arm. SAEs in the Asian safety population were all non-hematological (talazoparib: pericardial effusion, foot fracture, and cardiac tamponade; chemotherapy: pyrexia, pleural effusion, pathologic fracture, and localized edema). None of the Asian patients permanently discontinued treatment due to TEAEs, and there were no deaths due to TEAEs among the Asian safety population.
The most common nonhematologic AEs among patients in the Asian safety population were nausea (talazoparib arm, n=11 [47.8%]; chemotherapy arm, n=4 [40.0%]); and fatigue (talazoparib arm, n=10 [43.5%]; chemotherapy arm, n=2 [20.0%]), occurring in a similar proportion of patients to the overall safety population (Table 6).
Among patients in the Asian subgroup, neutropenia was the most common hematologic TEAE reported in the talazoparib arm, but was less frequent with talazoparib than with chemotherapy (9/23 [39.1%] vs. 5/10 [50.0%]) (Table 6). Most neutropenia TEAEs in the talazoparib arm (6/9 patients, 66.7%) and all neutropenia TEAEs in the chemotherapy arm (5/5 patients, 100.0%) were grade 3/4 in intensity. In patients treated with talazoparib, grade 3/4 anemia and thrombocytopenia were both less common in the Asian subgroup than in the overall safety population (Table 6). Median time to onset of first treatment-emergent hematologic AE and median duration of treatment-emergent hematologic AEs is shown in S1 Fig.
Grade 3/4 laboratory abnormalities for hemoglobin, neutrophils, and platelets reported for talazoparib in the Asian subgroup corresponded with the hematologic TEAEs reported for anemia, neutropenia, and thrombocytopenia, and generally occurred at a similar frequency to that observed in the overall safety population (Table 7). The use of red blood cell (RBC) transfusions in the talazoparib and chemotherapy arms was 17.4% and 10.0%, respectively, in the Asian subgroup, and 38.1% and 5.6%, respectively, in the overall population. No patients in the Asian subgroup received platelet transfusions; in the overall safety population, platelet transfusions were performed in 3.1% of patients in the talazoparib arm and no patients in the chemotherapy arm.
No grade 3/4 post-baseline chemistry toxicities were observed among talazoparib-treated patients in the Asian subgroup.
DiscussionDifferences in the epidemiology, and tumor and host biology of breast cancer between Asian and Western patient populations may have implications for clinical management, particularly with regard to the tolerability of targeted treatments [15,23]. The current analysis, including a small number of patients (n=33) enrolled at sites in Asia (Korea and Taiwan) as part of the EMBRACA study, suggests that talazoparib is effective in Asian patients with HER2-negative advanced breast cancer with gBRCA1/2 mutation, showing a median PFS by blinded central review of 9.0 months (95% CI, 3.0 to 15.2), compared with 7.1 months (95% CI, 1.2 to not reached) for chemotherapy (HR, 0.74 [95% CI, 0.22 to 2.44]). A similar trend was also observed in the overall ITT population, where the median PFS among patients receiving talazoparib was longer compared with patients receiving chemotherapy (8.6 months [95% CI, 7.2 to 9.3] vs. 5.6 months [95% CI, 4.2 to 6.7; HR, 0.54 [95% CI, 0.41 to 0.71]; p < 0.001). As expected, median PFS in the talazoparib arms was similar in the Asian (9.0 months) and overall ITT populations (8.6 months) [8], suggesting that Asian and non-Asian regional patient populations similarly benefited from talazoparib treatment. In the chemotherapy arm, median PFS was longer in the Asian subgroup than in the overall EMBRACA ITT population (7.1 and 5.6 months, respectively) [8]. The reason for this is not clear; however, it most likely reflects the small number of patients in the Asian chemotherapy arm (n=10). In addition, a lower prevalence of visceral disease (60% [Asian] vs. 72% [overall]) and a relatively high prevalence of BRCA2 mutations (70% vs. 56%) may have favored chemotherapy-treated patients in the Asian versus overall ITT population. While BRCA1 mutation carriers are more likely to develop triple-negative breast cancer (TNBC), BRCA2 mutation carriers are more likely to develop hormone receptor-positive disease, which is generally associated with more favorable outcomes to chemotherapy than TNBC [24,25]. Patients enrolled at Asian sites also tended to be younger that those in the overall ITT population (60% vs. 47% were aged < 50 years).
The ORR was higher in the talazoparib arm than the chemotherapy arm in the Asian subgroup, similar to findings in the overall EMBRACA population [8]. Likewise, the duration of objective response was longer for talazoparib than for chemotherapy across both the Asian and overall populations, with a particularly long duration of response observed among talazoparib-treated patients in the Asian subgroup (median, 9.5 months). Median OS was comparable between the talazoparib and chemotherapy arms for both the Asian and overall ITT populations [26].
One of the concerns regarding ethnic differences in drug safety and tolerability in patients with breast cancer is that women in East Asia tend to have a lower body mass, which may contribute to greater toxicity with targeted agents that are typically administered at fixed doses [15]. In the current analysis, patients in the Asian subgroup had a lower median body weight compared with the overall ITT population (talazoparib, 58.6 kg vs. 65.6 kg, respectively; chemotherapy; 52.0 kg vs. 66.0 kg, respectively). Despite this, talazoparib was generally well tolerated among patients in the Asian subgroup, with an overall similar frequency of TEAEs to that observed in the overall safety population, but, interestingly, with a lower frequency of grade 3/4 TEAEs, SAEs, and TEAEs leading to dose reduction or permanent discontinuation [8]. Although the median duration of talazoparib treatment was similar in the Asian and overall populations (5.7 months and 6.1 months, respectively) [8], fewer patients in the Asian subgroup received dose reductions compared with the overall population, inferring that Asian patients were able to maintain the 1 mg/day dose for longer than non-Asian patients. Compared with the overall safety population [8], the frequencies of grade 3/4 anemia and thrombocytopenia with talazoparib were also numerically lower in the Asian safety population, which translated into a lower rate of RBC and platelet transfusions. In a population pharmacokinetic analysis of talazoparib in patients with advanced cancers, oral talazoparib clearance was 24.7% higher, corresponding to a 20% lower exposure in Asian than in non-Asian patients [27]. Because the maximum tolerated dose of talazoparib (1 mg/day) was determined in a phase I study that included Asian patients, no dose adjustment is recommended in Asian patients, and 1 mg/day is the recommended starting dose, regardless of race or ethnicity [7,9,27]. Nonetheless, the higher clearance and lower exposure of talazoparib, as well as the younger Asian study population compared with the overall ITT population may have enabled patients in the Asian subgroup to better tolerate talazoparib and thus reduced the need for dose reductions compared with non-Asian patients.
A subgroup analysis of the randomized phase III trial, OlympiAD, comparing olaparib monotherapy with chemotherapy (capecitabine, eribulin, or vinorelbine) in patients with HER2-negative advanced breast cancer and a gBRCA1/2 mutation was conducted using data from patients enrolled in Asia (China, Japan, Korea, and Taiwan) [18]. Similar to our findings, the olaparib arm achieved numerically longer PFS by blinded central review versus the chemotherapy arm, and efficacy and safety findings were consistent with the overall OlympiAD population [18]. In contrast with the current analysis, the OlympiAD subgroup analysis found that grade ≥ 3 anemia in the olaparib arm occurred at a slightly higher frequency in the Asian subgroup than in the overall study population [18]. However, this is in line with the comparatively low frequency of grade ≥ 3 anemia in the overall OlympiAD population versus that in the EMBRACA study [8,18] and reflective of the different toxicity profiles observed with different PARP inhibitors across clinical trial populations [28]. Overall, the results of the current analysis, together with findings from the OlympiAD subgroup analysis [18], suggest that the PARP inhibitors talazoparib and olaparib are effective in Asian patients with HER2-negative advanced breast cancer and a gBRCA1/2 mutation, with comparable efficacy and safety to that in non-Asian patients. There were no confirmed cases of myelodysplastic syndrome. As reported with the primary data analysis, one case of acute myeloid leukemia (AML) was reported in a patient who received capecitabine [8], and a previously unreported case of AML occurred in a patient who received talazoparib (reported after the data cutoff of the safety data herein presented), both non-Asian [26].
A limitation of the current study is the small sample size of both treatment arms in the Asian subgroup. Safety evaluation may also be subject to reporting by the investigators, however, underreporting is unlikely and, if it occurred, it is anticipated to be similar to that in the overall EMBRACA safety population. Patients from Korea and Taiwan may not be necessarily representative of patients from the rest of Asia. Moreover, correlation of outcomes with other patient factors and/or biomarkers was not explored due to the limited sample size of this subset, thus limiting the conclusions that can be drawn regarding the importance of other clinical factors.
In conclusion, with all the limitations determined by the small sample size, the findings of this subgroup analysis still provide valuable information on the use of talazoparib in patients from Asian countries. Talazoparib numerically improved efficacy outcomes versus chemotherapy in patients with HER2-negative locally advanced/metastatic breast cancer with a gBRCA1/2 mutation enrolled at Asian sites in the EMBRACA study. Talazoparib was also generally well tolerated in the Asian subgroup, and AEs were manageable and consistent with the known safety profile of talazoparib. Furthermore, the lower talazoparib exposure in Asian patients suggests that they can remain on the 1-mg dose for longer than other patient populations. The consistency of our findings with the results in the overall EMBRACA population indicates that previously reported findings with talazoparib are also relevant to Asian patients. Nevertheless, there is a need to include more patients from Asia in breast cancer global clinical trials to generate solid efficacy and safety data that are specific to Asian patient populations, as underlined in previous works [15,18].
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The EMBRACA study was performed in accordance with the Declaration of Helsinki and International Council for Harmonisation Guidelines on Good Clinical Practice. The protocol was approved by an independent ethics committee, and all patients provided written informed consent. Conflicts of Interest Kyung-Hun Lee reports honoraria from Roche and AstraZeneca, and has participated in advisory boards for Bayer, Ono Pharmaceutical, Samsung Bioepis, Roche, Eisai, and AstraZeneca. Sung-Bae Kim reports research funding from Novartis, Sanofi-Aventis, and DongKook Pharm Co., and has participated as a consultant in advisory boards for Novartis, AstraZeneca, Lilly, Dae Hwa Pharmaceutical Co. Ltd, ISU Abxis, and Daiichi-Sankyo. Joohyuk Sohn reports research grant/funding from MSD, Roche, Novartis, AstraZeneca, Lilly, Pfizer, Bayer, GSK, CONTESSA, and Daiichi-Sankyo. Annabel Goodwin reports honoraria from AstraZeneca and Pfizer for participation in advisory boards. Tiziana Usari and Silvana Lanzalone are employees of Pfizer. Seock-Ah Im reports research funding from AstraZeneca, Eisai, Roche, Pfizer, and Daewoong Pharm Co., and has participated as a consultant in advisory boards for AstraZeneca, Amgen, Hanmi, Eisai, GSK, Idience, Lilly, MSD, Novartis, Daiichi-Sankyo, Roche, and Pfizer. Author Contributions Collected the data: Lee KH, Sohn J, Goodwin A, Im SA, Kim SB. Contributed data or analysis tools: Lee KH, Sohn J, Goodwin A, Usari T, Lanzalone S, Im SA, Kim SB. Performed the analysis: Lee KH, Sohn J, Goodwin A, Usari T, Lanzalone S, Im SA, Kim SB. Wrote the paper: Lee KH, Sohn J, Goodwin A, Usari T, Lanzalone S, Im SA, Kim SB. AcknowledgmentsThe authors would like to thank the study participants, their families, the investigators, and staff who participated in this study.
Medical writing support, under the direction of the authors, was provided by Katharine Howe of CMC AFFINITY, McCann Health Medical Communications, with funding from Pfizer, in accordance with Good Publication Practice (GPP3) guidelines.
This work was sponsored by Medivation, which was acquired by Pfizer Inc. in September 2016 (grant number not applicable).
Fig. 1Patient disposition: Asian and EMBRACA ITT populations. From The New England Journal of Medicine, Litton JK et al, Talazoparib in patients with advanced breast cancer and a germline BRCA mutation, 379:753–63 [8]. Copyright © (2020) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. AE, adverse event; ITT, intent-to-treat; PD, progressive disease. a)Including patients who died, withdrew consent or were lost to follow-up, b)Preferred terms included anemia, neutropenia, thrombocytopenia, vomiting, fatigue, general physical health deterioration, mucosal inflammation, edema peripheral, accidental overdose, glioblastoma multiforme, metastases to meninges, cerebral hemorrhage, headache, transient ischemic attack, dyspnea, obstructive airways disorder, rash, and rash generalized. 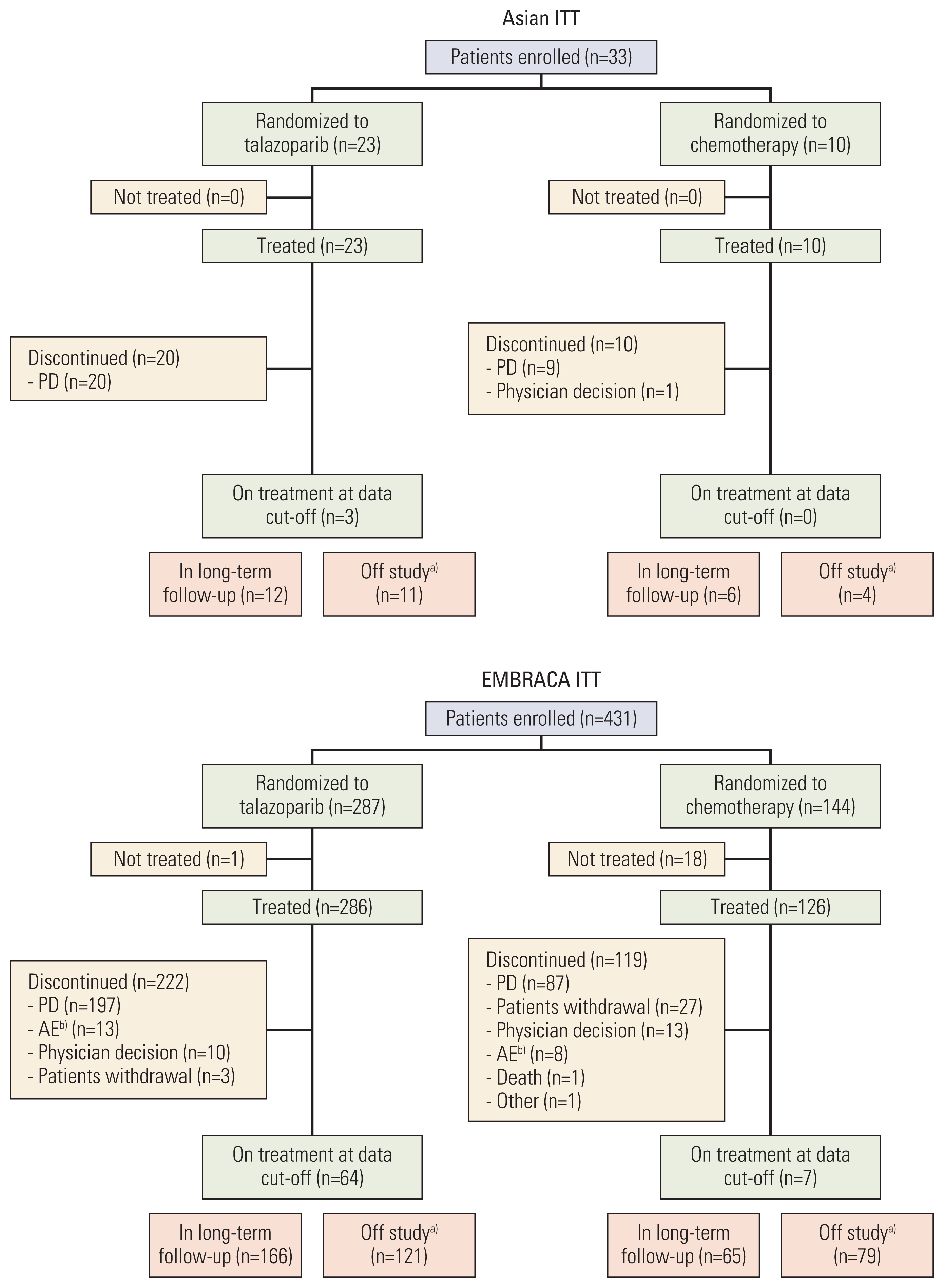
Fig. 2PFS by blinded central review (A, B) and investigator assessment (C, D) in the ITT populationsa). a)From The New England Journal of Medicine, Litton JK et al, Talazoparib in patients with advanced breast cancer and a germline BRCA mutation, 379:753–63 [8]. Copyright © (2020) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. CI, confidence interval; HR, hazard ratio; ITT, intent-to-treat; NR, not reached; PCT, physician’s choice of treatment. 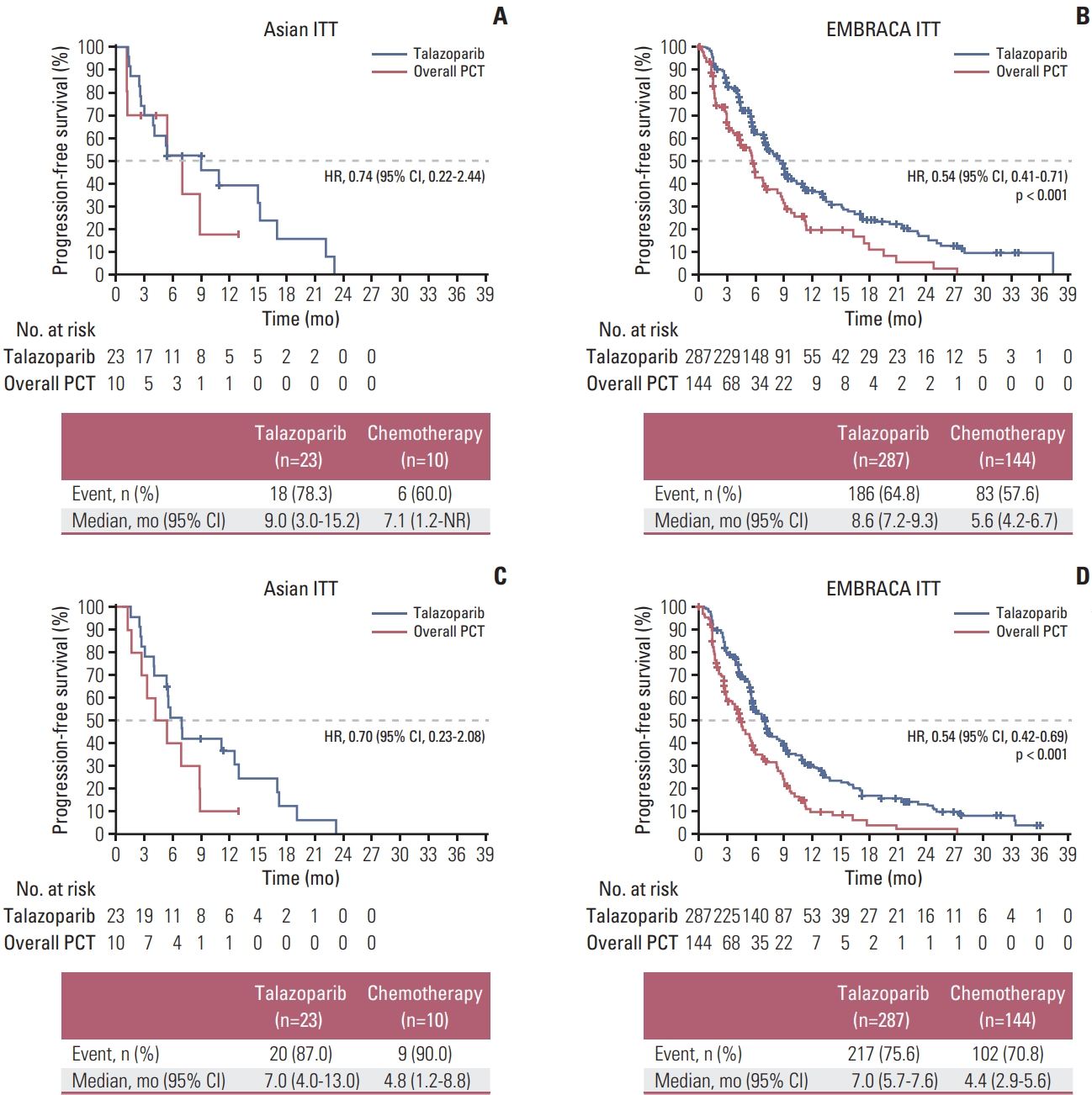
Table 1Baseline demographic characteristics: Asian ITT and EMBRACA ITT populations
Values are presented as number (%) unless otherwise indicated. ABC, advanced breast cancer; BRCA1/2, breast cancer susceptibility genes 1 or 2; HR, hormone receptor; ITT, intent-to-treat; TNBC, triple-negative breast cancer. a) From The New England Journal of Medicine, Litton JK et al., Talazoparib in patients with advanced breast cancer and a germline BRCA mutation, 379:753–63 [8]. Copyright © (2020) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society, Table 2ORR in patients with measurable disease at baseline
CI, confidence interval; CR, complete response; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease. a) From The New England Journal of Medicine, Litton JK et al., Talazoparib in patients with advanced breast cancer and a germline BRCA mutation, 379:753–63 [8]. Copyright © (2020) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society, Table 3OS in the ITT populations
a) From Litton et al. Ann Oncol. 2020;31:1526–35 [26]. Table 4Study drug exposure
a) From The New England Journal of Medicine, Litton JK et al., Talazoparib in patients with advanced breast cancer and a germline BRCA mutation, 379:753–63 [8]. Copyright © (2020) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society, Table 5Overall summary of TEAEs
a) From The New England Journal of Medicine, Litton JK et al., Talazoparib in patients with advanced breast cancer and a germline BRCA mutation, 379:753–63 [8]. Copyright © (2020) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society, Table 6Hematologic TEAEs and most common nonhematologic TEAEs (≥ 25% of patients)
a) From The New England Journal of Medicine, Litton JK et al., Talazoparib in patients with advanced breast cancer and a germline BRCA mutation, 379:753–63 [8]. Copyright © (2020) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society, b,c) Number of patients receiving talazoparib who permanently discontinued due to a grade 3/4 hematologic treatment-emergent adverse event: b)n=2; c)n=1 in overall safety, d) The majority of nonhematologic toxicities were grade 1 or 2; for the talazoparib arm, alopecia was reported only as grade 1 (17.4%), no grade 2 in the Asian safety population and mostly grade 1 (22.7%) in the EMBRACA safety population. Patients with multiple events for a given preferred term were counted only once for each preferred term. The anemia category includes preferred terms: anemia, decreased hemoglobin, decreased hematocrit. The neutropenia category includes preferred terms: neutropenia, decreased neutrophil count. The thrombocytopenia category includes preferred terms: thrombocytopenia, platelet count decreased. The leukopenia category includes preferred terms: leukopenia, white blood cell count decreased. The lymphopenia category includes preferred terms lymphopenia, lymphocyte count decreased. Table 7Patients with grade 3/4 post-baseline hematologic and chemistry toxicities References1. Javle M, Curtin NJ. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol. 2011;3:257–67.
2. Ashworth A. A synthetic lethal therapeutic approach: poly (ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785–90.
3. Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5:387–93.
4. Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152–8.
5. Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–99.
6. Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014;13:433–43.
7. de Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S, et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017;7:620–9.
8. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–63.
9. U.S. Food and Drug AdministrationTALZENNA (talazoparib) prescribing information [Internet]. New York: Pfizer; c2020. [cited 2020 Nov 19]. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=11046
10. European Medicines AgencyTALZENNA (talazoparib) Summary of product characteristics [Internet]. New York: Pfizer; c2019. [cited 2020 Nov 19]. Available from: https://www.ema.europa.eu/en/documents/product-information/talzenna-epar-product-information_en.pdf
11. Li G, Guo X, Tang L, Chen M, Luo X, Peng L, et al. Analysis of BRCA1/2 mutation spectrum and prevalence in unselected Chinese breast cancer patients by next-generation sequencing. J Cancer Res Clin Oncol. 2017;143:2011–24.
12. Lang GT, Shi JX, Hu X, Zhang CH, Shan L, Song CG, et al. The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer. 2017;141:129–42.
13. Kim H, Choi DH. Distribution of BRCA1 and BRCA2 mutations in Asian patients with breast cancer. J Breast Cancer. 2013;16:357–65.
14. Wen WX, Allen J, Lai KN, Mariapun S, Hasan SN, Ng PS, et al. Inherited mutations in BRCA1 and BRCA2 in an unselected multiethnic cohort of Asian patients with breast cancer and healthy controls from Malaysia. J Med Genet. 2018;55:97–103.
15. Yap YS, Lu YS, Tamura K, Lee JE, Ko EY, Park YH, et al. Insights Into breast cancer in the East vs the West: a review. JAMA Oncol. 2019;5:1489–96.
16. Bhaskaran SP, Huang T, Rajendran BK, Guo M, Luo J, Qin Z, et al. Ethnic-specific BRCA1/2 variation within Asia population: evidence from over 78 000 cancer and 40,000 non-cancer cases of Indian, Chinese, Korean and Japanese populations. J Med Genet. 2020. Sep. 22[Epub]. https://doi.org/10.1136/jmedgenet-2020-107299
17. Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, Fehrenbacher L, et al. Outcomes in clinically relevant patient subgroups from the EMBRACA study: talazoparib vs physician’s choice standard-of-care chemotherapy. JNCI Cancer Spectr. 2020;4:pkz085.
18. Im SA, Xu B, Li W, Robson M, Ouyang Q, Yeh DC, et al. Olaparib monotherapy for Asian patients with a germline BRCA mutation and HER2-negative metastatic breast cancer: OlympiAD randomized trial subgroup analysis. Sci Rep. 2020;10:8753.
19. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33.
20. Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians vs the US population. J Natl Cancer Inst. 2019;111:1298–306.
21. Bhoo-Pathy N, Yip CH, Hartman M, Uiterwaal CS, Devi BC, Peeters PH, et al. Breast cancer research in Asia: adopt or adapt Western knowledge? Eur J Cancer. 2013;49:703–9.
22. Hurvitz SA, Goncalves A, Rugo HS, Lee KH, Fehrenbacher L, Mina LA, et al. Talazoparib in patients with a germline BRCA-mutated advanced breast cancer: detailed safety analyses from the phase III EMBRACA trial. Oncologist. 2020;25:e439–50.
23. Kan Z, Ding Y, Kim J, Jung HH, Chung W, Lal S, et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018;9:1725.
24. Chen H, Wu J, Zhang Z, Tang Y, Li X, Liu S, et al. Association between BRCA status and triple-negative breast cancer: a meta-analysis. Front Pharmacol. 2018;9:909.
25. Shi J, Liu F, Song Y. Progress: targeted therapy, immunotherapy, and new chemotherapy strategies in advanced triple-negative breast cancer. Cancer Manag Res. 2020;12:9375–87.
26. Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee KH, Goncalves A, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020;31:1526–35.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||