AbstractPurposeWe recently reported on a randomized, open-label, phase 3 trial comparing pemetrexed-cisplatin chemotherapy followed by gefitinib maintenance therapy (PC/G) with gefitinib monotherapy in patients with non-small cell lung cancer (NSCLC). Here, we report on a post hoc subgroup analysis of that study assessing the demographics and disposition of the Korean patient subgroup, and comparing the tolerability of PC/G and gefitinib monotherapy and the tumor response with respect to epidermal growth factor receptor (EGFR) status.
Materials and MethodsPatients, who were ≥ 18 years, chemonaïve, Korean, light ex-smokers/never-smokers with advanced NSCLC, were randomly assigned (1:1) to PC/G or gefitinib monotherapy. Treatment-emergent adverse events (TEAEs) were graded, and tumor response was measured as change in lesion sum from baseline at best response. The study was registered with ClinicalTrials. gov, NCT01017874.
ResultsOverall, 111 Korean patients were treated (PC/G, 51; gefitinib, 60). Between-arm characteristics were balanced and similar to those of the overall population. Treatment discontinuations due to adverse events were low (PC/G: 1, 2.0%; gefitinib: 7, 11.7%). Overall, 92 patients (82.9%) reported ≥ 1 TEAE (PC/G, 44; gefitinib, 48); few patients (PC/G, 16; gefitinib, 7) reported severe TEAEs; the most frequent was neutropenia (PC/G arm) and elevated alanine aminotransferase (gefitinib arm). The lesion sum was decreased by PC/G treatment in most patients, regardless of EGFR mutation status, while gefitinib monotherapy reduced the lesion sum in EGFR-positive patients but had no effect in EGFR-negative patients.
IntroductionLung cancer is the main cause of cancer-related deaths in East Asia, accounting for one in four of all cancer-related deaths [1]. In Korea, an estimated 16,990 deaths in 2014 were caused by lung cancer, accounting for 22.7% of all cancer-related deaths [2]. The current first-line standard of care for advanced non-small cell lung cancer (NSCLC) is platinum-based doublet chemotherapy [3], although inhibitors of the epidermal growth factor receptor (EGFR) tyrosine kinase, such as gefitinib, are recommended in patients harboring activating EGFR mutations [4-6]. The phase 3 Iressa Pan-Asia Study (IPASS), which compared gefitinib monotherapy with combination chemotherapy as first-line therapy in East Asian patients, reported significantly longer progression-free survival (PFS) in patients who had EGFR mutations and were treated with gefitinib [7]. A subsequent phase 2 trial, in which combination chemotherapy followed by gefitinib or pemetrexed maintenance therapy was administered in East Asian patients with unknown EGFR status, reported longer PFS in patients receiving gefitinib than those receiving pemetrexed [8]. These findings may be attributed to the high frequency of EGFR mutations in East Asian patients with NSCLC [5,7,8].
The current randomized, open-label, phase 3 trial was designed for comparison of pemetrexed-cisplatin (PC) doublet chemotherapy followed by gefitinib maintenance therapy (PC/G) with gefitinib monotherapy in East Asian patients with NSCLC and unknown EGFR mutation status [9]. In the overall study population, there was no significant difference in PFS between treatment arms [9]. However, treatment response may differ in patients from various regions of East Asia or those with EGFR mutations that were not evident from the primary analysis. To further assess potential differences in treatment response in the East Asian population, we conducted a post hoc descriptive subgroup analysis of Korean patients from this phase 3 trial. The Korean subgroup comprised the largest proportion of patients in the original study. The aims of the subgroup analysis were to assess demographics and disposition of the Korean patients randomized in the study, compare the tolerability of PC/G therapy with gefitinib monotherapy in this subgroup, and assess the tumor response with respect to EGFR status for each treatment arm.
Materials and MethodsThis study was a post hoc subgroup analysis of data from a randomized, open-label, phase 3 clinical trial (NCT0101 7874). Findings for the primary objective, which was to compare PFS in patients treated with PC/G with those treated with gefitinib monotherapy, were reported previously by Yang et al. [9]. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki; all patients provided informed consent. Patient eligibility criteria included age ≥ 18 years, chemonaïve, East Asian, and light ex-smokers/never-smokers with advanced NSCLC. Patients were assigned (1:1) to receive either PC/G therapy (PC [P, 500 mg/m2; C, 75 mg/m2] for six 21-day induction cycles, then gefitinib [250 mg/day] maintenance therapy) or gefitinib monotherapy (G [250 mg/day], administered until progression, discontinuation, or death). Treatment-emergent adverse events (TEAEs; possibly drug-related) and serious adverse events were classified according to MedDRA (ver. 15.1) and graded. Safety analyses were performed on the safety population, which included all patients who received ≥ 1 dose of the study drug. Tumor response, conducted on the tumor response-qualified population (patients with lesion measurements taken at baseline and at least 1 other time point), was measured as change in lesion sum from baseline at best response. Change in lesion sum was determined as the change from baseline in the sum of the largest diameter of each lesion (up to a maximum of 10 lesions per patient). Analysis of patient tissue was performed retrospectively for EGFR mutations.
Results1. Patient dispositionA total of 253 patients were enrolled in the phase 3 study, 120 of whom were enrolled in Korea (Fig. 1). Of the 120 Korean patients enrolled in the study, 114 patients were randomly assigned to treatment: 54 patients were assigned to PC/G (3 of whom were not treated); 60 patients were assigned gefitinib monotherapy. Thirty-three patients in the PC/G arm received gefitinib maintenance therapy and 28 patients in the gefitinib arm received more than six cycles of treatment.
2. Patient demographicsBaseline patient demographics were balanced between arms and similar to those reported for the overall study population (Table 1) [9]. All Korean patients had adenocarcinoma, most were never-smokers (93.9%), had stage IV disease (91.2%), and had a Eastern Cooperative Oncology Group (ECOG) performance status of 1 (57.0%) (Table 1).
3. Treatment durationThe median number of treatment cycles completed overall was 7.0 and 6.0 for PC/G and gefitinib monotherapy, respectively. The median number of treatment cycles was 6.0 for both treatment arms during the induction period, and 4.0 and 7.5 for PC/G and gefitinib monotherapy, respectively, during the maintenance period.
4. Response to therapy by EGFR statusThe change in lesion sum from baseline was calculable for 47 patients in the PC/G arm and 53 patients in the gefitinib arm. Lesion sum was reduced by treatment with PC/G in the majority of Korean patients with NSCLC, regardless of EGFR mutation status (Figs. 2A and 3A). In this selected population, lesion sum was reduced by gefitinib monotherapy in the subgroup of Korean patients who were confirmed as EGFR positive (n=8) (Figs. 2B and 3B). In contrast, gefitinib monotherapy had no positive effect on lesion sum in the subgroup of Korean patients who were EGFR negative (n=3) (Figs. 2B and 3B).
5. Adverse eventsOverall, 92 of 111 patients (82.9%) reported at least 1 TEAE during treatment. Treatment discontinuation due to adverse events (AEs) was low (PC/G, 1/51 [2.0%]; gefitinib, 7/60 [11.7%]). Few patients (PC/G, 16/51 [31.4%]; gefitinib, 7/60 [11.7%]) reported severe (grades 3-4) TEAEs during the overall treatment period; the most frequent was neutropenia in the PC/G treatment arm and elevated alanine aminotransferase in the gefitinib treatment arm. No patients reported grade 5 TEAEs (Table 2).
ConclusionPatient demographics and AEs in Korean patients with NSCLC were similar to those reported in the overall study population [9]. A slightly higher proportion of Korean patients experienced grades 3-4 hematologic TEAEs following PC/G treatment compared with the overall population, but, overall, both PC/G and gefitinib monotherapy were well tolerated in Korean patients with NSCLC. Although a limited number of samples were available for EGFR testing, post hoc analysis of tumor response based on EGFR mutation status showed that PC/G treatment consistently reduced lesion sum in patients with and without EGFR mutations, but gefitinib monotherapy had no effect in patients with EGFR wild-type. The findings of this Korean patient subgroup analysis confirmed that PC/G and gefitinib monotherapy showed favorable tolerability in patients with advanced NSCLC; however, such patients with EGFR wild-type may not benefit from gefitinib monotherapy.
Conflicts of InterestXW, JSK, and MO are employees of Eli Lilly and Company. MO owns shares in Eli Lilly Pty Ltd. JH Kang is an Advisory Board member for Eli Lilly and Company. MJA has consulted/advised for Eli Lilly and Company. DWK has consulted/advised for Eli Lilly and Company. KP has consulted/advised for Astellas, Astra-Zeneca, AVEO, and Boehringer. EKC, JH Kim, and SWS have no conflicts of interest to disclose. This study was sponsored by Eli Lilly and Company, manufacturer/licensee of pemetrexed (Alimta®). Medical writing assistance was provided by Rose Boutros, PhD, and Julie Ely, PhD, CMPP, of ProScribe - Envision Pharma Group, and was funded by Eli Lilly. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP2). Fig. 1.Disposition of Korean patients with non-small cell lung cancer treated with PC/G or gefitinib monotherapy. PC/G, pemetrexed-cisplatin/gefitinib. 
Fig. 2.Waterfall plots of percent change in lesion sum from baseline at best response by epidermal growth factor receptor(EGFR) status in Korean patients treated with pemetrexed-cisplatin/gefitinib (A) and gefitinib monotherapy (B). 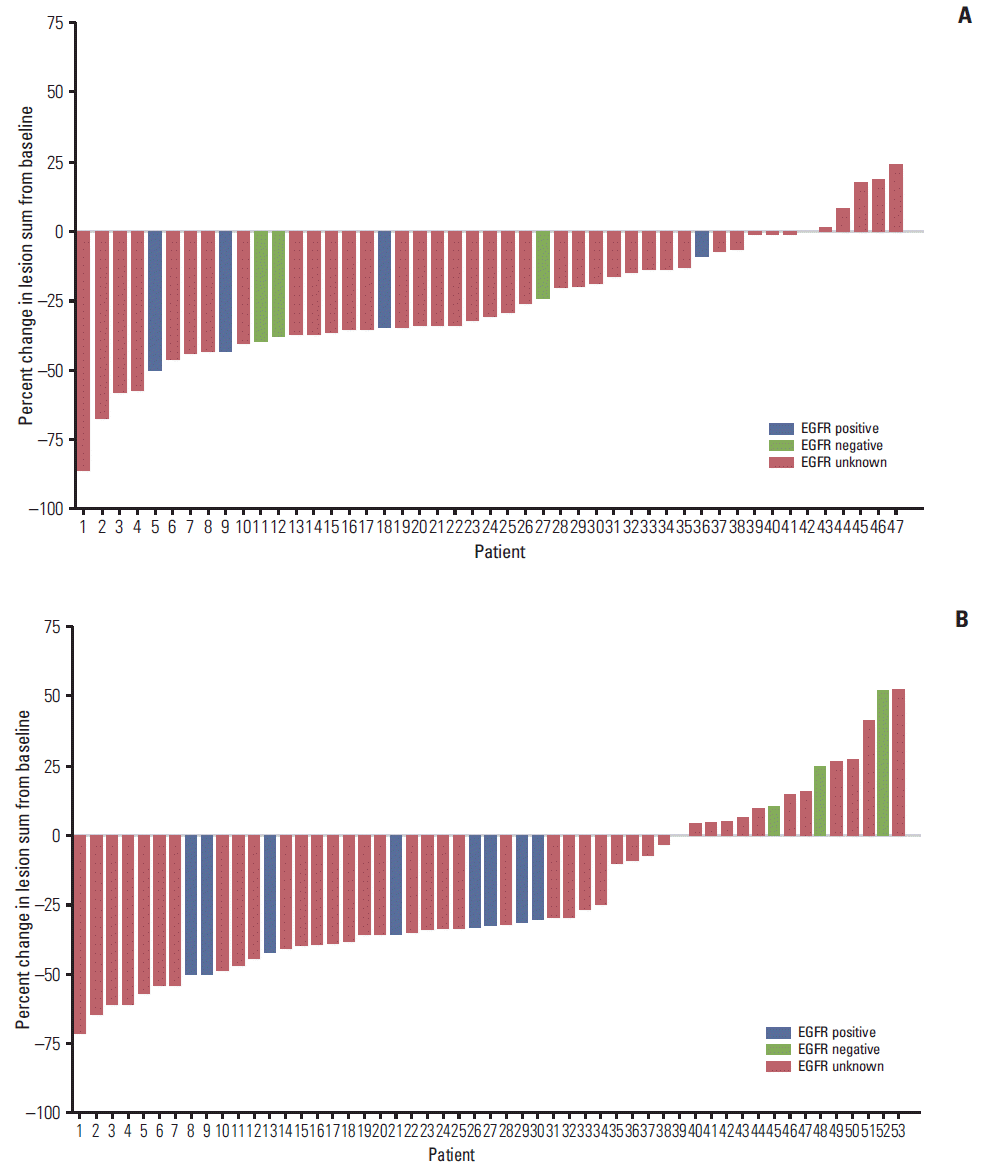
Fig. 3.Spider plots of percent change in lesion sum from baseline at best response by epidermal growth factor receptor (EGFR) status in Korean patients treated with pemetrexed-cisplatin/gefitinib (A) and gefitinib monotherapy (B). 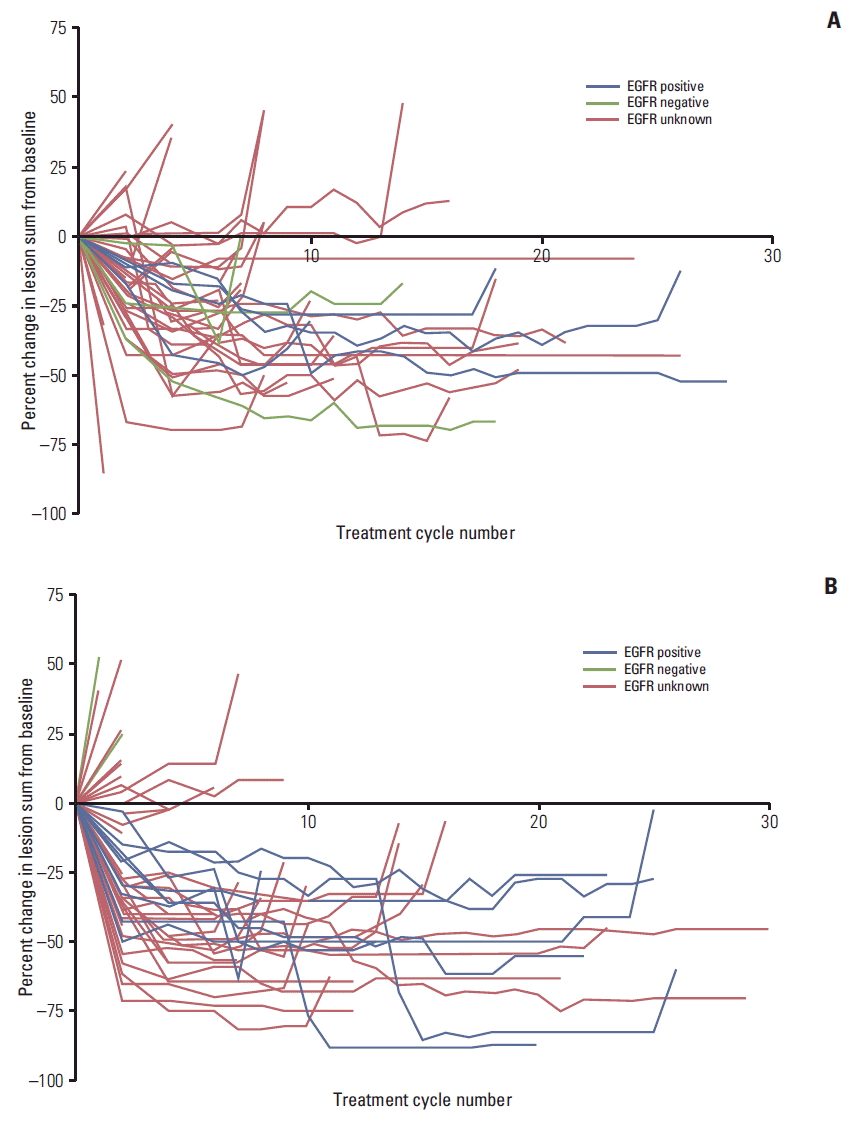
Table 1.Baseline patient demographics for the Korean patient subgroup Table 2.Adverse events reported in at least 5% of Korean patients in either treatment arm during the induction phase of treatment
References1. Ferlay J, Soerjomataram I, Ervik M, Forman D, Bray F, Dikshit R, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon: International Agency for Research on Cancer; 2013 [cited 2015 Mar 24]. Available from: http://globocan.iarc.fr
2. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Prediction of cancer incidence and mortality in Korea, 2014. Cancer Res Treat. 2014;46:124–30.
3. D'Addario G, Fruh M, Reck M, Baumann P, Klepetko W, Felip E, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v116–9.
4. Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27:6251–66.
5. Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137:828–60.
6. Socinski MA, Evans T, Gettinger S, Hensing TA, Sequist LV, Ireland B, et al. Treatment of stage IV non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e341S–68S.
7. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57.
8. Ahn MJ, Yang JC, Liang J, Kang JH, Xiu Q, Chen YM, et al. Randomized phase II trial of first-line treatment with pemetrexedcisplatin, followed sequentially by gefitinib or pemetrexed, in East Asian, never-smoker patients with advanced non-small cell lung cancer. Lung Cancer. 2012;77:346–52.
9. Yang JC, Kang JH, Mok T, Ahn MJ, Srimuninnimit V, Lin CC, et al. First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian patients with locally advanced or metastatic non-squamous non-small cell lung cancer: a randomised, phase 3 trial. Eur J Cancer. 2014;50:2219–30.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||