AbstractAdvances in oncology research have led to identification of tumor-specific biomarkers, some of which are important predictive indicators and ideal targets for novel therapeutics. One such biomarker in non-small cell lung cancer (NSCLC) is the epidermal growth factor receptor (EGFR). Patients with NSCLC who harbor an activating EGFR mutation show a more favorable response to treatment with an EGFR inhibitor, such as gefitinib, erlotinib, or afatinib, than to chemotherapy. The prevalence of EGFR mutations in East Asian patients is higher than that in other populations, and in some clinical settings, patients have been treated with EGFR inhibitors based on clinicopathologic characteristics with no information on EGFR status. However, based on results from a series of studies in which East Asian patients with advanced non-squamous NSCLC were treated with EGFR inhibitors alone or in combination with standard chemotherapy, this may not be the best practice because EGFR mutation status was found to be a key predictor of outcome. Data from these studies highlight the necessity of EGFR testing in determining the most suitable treatment for patients with advanced or metastatic NSCLC.
IntroductionAdvances in cancer research over the last decade have led to a drive towards personalized medicine. This has been largely facilitated by the identification of tumor-specific biomarkers and advances in the methods used to detect them [1,2]. Lung cancer is an ideal model of how biomarkers have been identified and used in targeted therapy for specific patient subgroups who harbor the biomarker.
Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancers, and patients are usually diagnosed in the advanced stages of disease [3]. A number of genetic alterations have been identified in patients with NSCLC, and the prevalence of these alterations differs depending on the patient’s clinicopathologic features, including ethnicity, gender, smoking history, and histological subtype [4]. Mutation of the key tyrosine kinase gene epidermal growth factor receptor (EGFR), involved in growth factor receptor signaling, is commonly observed in tumor samples from patients with NSCLC [1,4]. A number of small molecule tyrosine kinase inhibitors (TKIs) that specifically target EGFR in the treatment of NSCLC have been developed.
Gefitinib, the first small molecule inhibitor of EGFR, was found to significantly prolong progression-free survival (PFS) in a subset of patients with advanced NSCLC. Subsequent findings showed that these patients had activating somatic mutations in the EGFR gene [5,6]. In an analysis of several studies involving treatment with the EGFR-targeted TKIs, gefitinib and erlotinib, resulted in a response to therapy in approximately 68% and 11% of patients who tested positive and negative (hereafter referred to as EGFR-positive and EGFR-negative) for activating EGFR mutations, respectively [4]. Correlation between EGFR mutations and enhanced response to TKI therapy has been verified by a number of randomized trials [7-13] including the Iressa Pan Asia Study (IPASS). In general, patients harboring EGFR mutations have a longer PFS with EGFR TKI therapy compared to chemotherapy, and show a more favorable response to EGFR TKI therapy than patients without EGFR mutations. Therefore, given that more than half of patients with NSCLC in East Asia who are non-smokers and have adenocarcinoma histology harbor EGFR mutations [14], it has become common practice in some Asian countries (where EGFR mutation testing is readily available and/or subsidized) to treat patients based on their EGFR status. Yet, in some clinical practices, this subgroup of patients is still treated with TKIs without prior testing for EGFR status because physicians are reluctant to delay the start of treatment or because sufficient tumor tissue may not be available. However, as noted above, EGFR-negative patients do not respond as well to TKI therapy as they do to standard chemotherapy, and, therefore, have inferior outcomes; thus, this may not be the best practice [13].
Body TextHere we report on experience gained from a series of studies conducted predominantly in East Asia and highlight some of the key findings and major limitations associated with determining EGFR status in patients with non-squamous NSCLC. The value of EGFR mutation status in predicting treatment outcomes was examined in a series of studies on East Asian patients with advanced NSCLC, in which the effect of EGFR TKI therapy, alone or in combination with standard chemotherapy, on treatment outcomes was examined in EGFR-positive and EGFR-negative patient subgroups (Table 1, Figs. 1 and 2) [15-17]. In a phase 2 randomized controlled trial involving 240 non-smoking patients with non-squamous NSCLC, of which 133 were East Asian, pemetrexed and erlotinib in combination were compared to either agent alone in the second-line treatment setting [15]. Collection of samples for EGFR testing was optional. As a result, in the East Asian population, EGFR status was available for only 31 patients, 19 of whom (61%) were EGFR positive, as expected by the clinical selection criteria. In these EGFR-positive patients from East Asia, patients treated with erlotinib had longer PFS than those treated with pemetrexed (Table 1, Fig. 1A) [15]. In contrast, in EGFR-negative patients, PFS was generally longer in patients treated with erlotinib in combination with pemetrexed than in those treated with either agent alone (Table 1, Fig. 1A) [15]. No obvious difference in change in lesion sum from baseline at best response was observed between treatment arms (Fig. 1B).
In a subsequent, open-label, phase 3 randomized trial involving 236 never-smoker East Asian patients with NSCLC, pemetrexed-cisplatin doublet chemotherapy as first-line treatment followed by gefitinib maintenance therapy was compared to gefitinib monotherapy [16]. Again, tissue collection for EGFR status was recommended but not mandatory, and the majority of tissue biopsies provided were too small for EGFR analysis. As a result, EGFR status was available for only 74 patients, 50 of whom (68%) were EGFR-positive. In EGFR-positive patients, there was no difference in PFS between treatment arms (Table 1, Fig. 2A) [16]. However, in EGFR-negative patients, patients receiving gefitinib monotherapy had shorter PFS compared to patients receiving chemotherapy followed by gefitinib maintenance (Fig. 2A) [16]. The beneficial effect of chemotherapy followed by gefitinib maintenance therapy in EGFR-negative patients was further evident by a reduction in the lesion sum from baseline at best response in these patients compared with patients receiving gefitinib monotherapy, in whom the lesion sum increased (Fig. 2B). These studies identified EGFR mutation status as a key predictor of outcome in East Asian never-smoker patients with non-squamous NSCLC and further confirmed that treating East Asian patients with NSCLC with EGFR TKIs is not necessarily beneficial in the absence of EGFR mutations [13,15-18]. Therefore, the findings from these studies highlight the potential benefits of consistent testing for EGFR status before determining the optimal treatment regimen for patients with NSCLC.
Testing for EGFR status is primarily restricted by challenges in specimen collection. In the phase 2 study by Lee et al. [15], fewer than 25% of patients provided samples for EGFR testing, approximately 20% of which were not evaluable. In the phase 3 study by Yang et al. [16] conducted 2 years later, when there was an increased awareness of the need for EGFR testing, a greater proportion (60%) of patients provided samples. However, almost 50% of these samples were not evaluable. Although there was an increased awareness of the importance of EGFR testing during the phase 3 study [16], the frequency of testing could still be improved to allow more patients to benefit from the most appropriately selected treatment. Due to the invasive nature of obtaining tissue biopsies, the limited availability of tissue for testing continues to hinder progress. In addition, greater efforts in sampling can be made to improve the proportion of evaluable samples. In cases where tissue samples are available, further care is needed in obtaining good-quality samples at the time of the first diagnostic biopsy and in preparation, storage, and testing of the samples.
ConclusionBased on the findings of the studies discussed above, it has become evident that physicians should test for EGFR mutation status, even in patients from East Asia, before deciding on the most appropriate treatment for patients with NSCLC. This is important because the treatment regimen cannot be optimized for patients with unknown EGFR status, which could compromise patient outcomes. In the phase 2 and phase 3 studies discussed here [15,16], EGFR status was tested retrospectively. Only a few patients provided samples and among those who did, even fewer samples yielded results. Thus, very low numbers were available to validate the clinical outcomes and enable accurate interpretation of the study findings. Molecular testing of biopsy samples for EGFR status is now being performed more frequently in clinical practice. However, still fewer than half of patients are providing samples and the sample quality may not be ideal for molecular testing. In light of the clinically relevant differences in response to TKI therapy and chemotherapy in patients with NSCLC who are EGFR-positive and EGFR-negative [13,15-17], patients should be tested for EGFR status before deciding on the most effective treatment regimen. Biomarker testing is becoming more important in clinical practice. Key challenges that investigators will need to overcome in the future include obtaining sufficient tumor tissue and development of more sensitive and more robust methods for testing.
Conflicts of InterestThis study was sponsored by Eli Lilly and Company, manufacturer/licensee of pemetrexed (Alimta). Eli Lilly and Company was involved in the design and preparation of the manuscript. RC, XW, and MO are employees of Eli Lilly and Company. RC and MO own shares in Eli Lilly Pty Ltd. DHL has received honoraria from Eli Lilly and Company as a member of an advisory board. VS has no relevant conflicts of interest to declare. Medical writing assistance was provided by Rose Boutros, PhD and Rebecca Lew, PhD, CMPP of ProScribe — Envision Pharma Group, and was funded by Eli Lilly. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP2). Fig. 1.Waterfall plots of progression-free survival (A) and percentage change in lesion sum from baseline at best response (B) by epidermal growth factor receptor (EGFR) status in East Asian patients with non-small cell lung cancer who were treated with erlotinib monotherapy, pemetrexed monotherapy, or pemetrexed/erlotinib (unpublished data from Lee et al. [17]). (B) Change in the lesion sum was not calculable for one EGFR-negative patient in the pemetrexed treatment group. 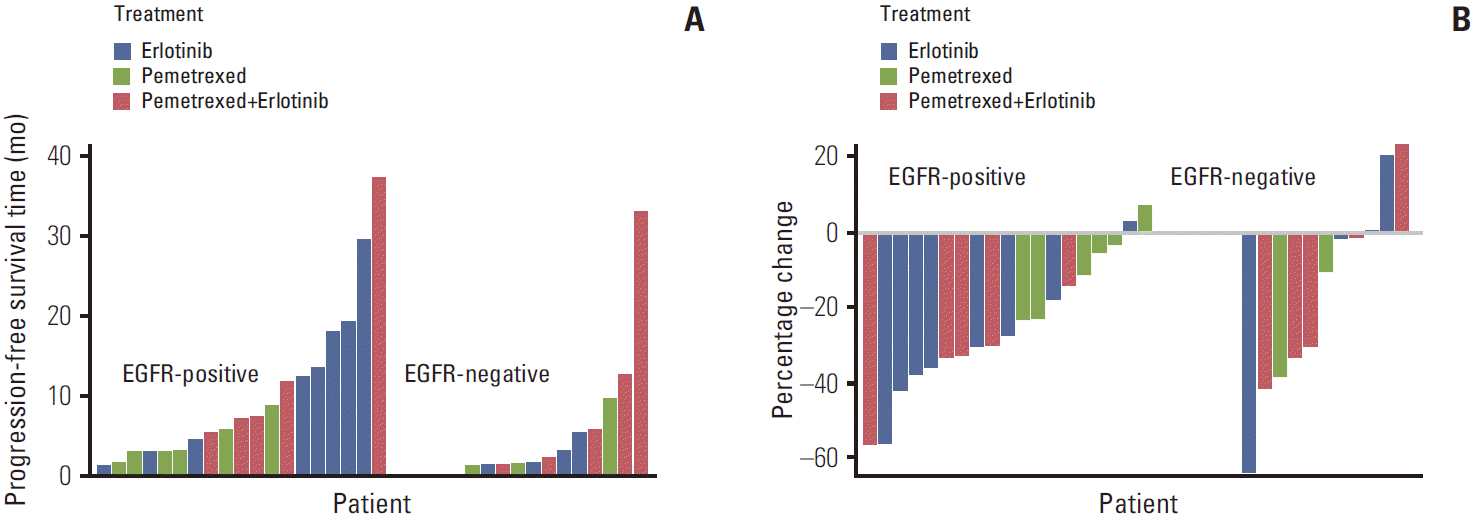
Fig. 2.Waterfall plots of progression-free survival (A) and percentage change in lesion sum from baseline at best response (B) by epidermal growth factor receptor (EGFR) status in East Asian patients with non-small cell lung cancer who were treated with gefitinib monotherapy or pemetrexed-cisplatin/gefitinib maintenance therapy (unpublished data from Yang et al. [16]). (B) Change in the lesion sum was not calculable for two EGFR-positive patients in the pemetrexed-cisplatin/gefitinib treatment group and four EGFR-negative patients in the gefitinib monotherapy group. 
Table 1.
EGFR, epidermal growth factor receptor; PE, pemetrexed-erlotinib; E, erlotinib; P, pemetrexted; PC/G, pemetrexed-cisplatin/gefitinib; G, gefitinib; PFS, progression-free survival; ND, not determined; TRR, tumor response rate; DCR, disease control rate. p-values derived from Wilcoxon test for PFS and Fisher exact test for TRR and DCR. a) p-values for PFS were not determined in Lee et al. [17] because the number of patients in each group was too low, References1. Brega E, Brandao G. Non-small cell lung carcinoma biomarker testing: the pathologist's perspective. Front Oncol. 2014;4:182.
2. Ulivi P, Zoli W, Capelli L, Chiadini E, Calistri D, Amadori D. Target therapy in NSCLC patients: relevant clinical agents and tumour molecular characterisation. Mol Clin Oncol. 2013;1:575–81.
3. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94.
4. Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137:828–60.
5. Miller VA, Kris MG, Shah N, Patel J, Azzoli C, Gomez J, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:1103–9.
6. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39.
7. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8.
8. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8.
9. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as firstline treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46.
10. Yang JC, Hirsh V, Schuler M, Yamamoto N, O'Byrne KJ, Mok TS, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3342–50.
11. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42.
12. Garassino MC, Borgonovo K, Rossi A, Mancuso A, Martelli O, Tinazzi A, et al. Biological and clinical features in predicting efficacy of epidermal growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis. Anticancer Res. 2009;29:2691–701.
13. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57.
14. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154–62.
15. Lee DH, Lee JS, Kim SW, Rodrigues-Pereira J, Han B, Song XQ, et al. Three-arm randomised controlled phase 2 study comparing pemetrexed and erlotinib to either pemetrexed or erlotinib alone as second-line treatment for never-smokers with nonsquamous non-small cell lung cancer. Eur J Cancer. 2013;49:3111–21.
16. Yang JC, Kang JH, Mok T, Ahn MJ, Srimuninnimit V, Lin CC, et al. First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian patients with locally advanced or metastatic non-squamous non-small cell lung cancer: a randomised, phase 3 trial. Eur J Cancer. 2014;50:2219–30.
17. Lee DH, Lee JS, Wang J, Hsia TC, Wang X, Kim J, et al. Pemetrexed-erlotinib, pemetrexed alone, or erlotinib alone as second-line treatment for East Asian and non-East Asian never-smokers with locally advanced or metastatic nonsquamous non-small cell lung cancer: exploratory subgroup analysis of a phase II trial. Cancer Res Treat. 2014;Nov. 24[Epub]. http://dx.doi.org/10.4143/crt.2014.051
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||