AbstractPurposeManagement of gastroenteropancreatic (GEP) neuroendocrine tumors with liver metastases (NETLM) presents many clinical challenges. Assessment of the extent of disease and primary tumor site is crucial for management. In this study, we investigated the primary tumor sites and prognostic factors in GEP NETLM among Korean patients.
Materials and MethodsWe reviewed the medical records of 72 Korean patients diagnosed with GEP NETLM between January 1999 and May 2013, focusing on their clinical and pathologic characteristics.
ResultsThe most frequently encountered primary tumor sites were the pancreas (n=25, 35%), stomach (n=8, 11%), gall bladder (n=4, 6%) and rectum (n=3, 4%). Twenty-five patients (35%) had occult primary tumor. Twelve patients (17%) had histological grade G1 tumors, 30 patients (42%) had G2 tumors, and 30 patients (42%) had G3 tumors. The mean follow-up period after histological confirmation of hepatic metastases was 11.30±2.44 months for G3 tumors, 19.67±4.09 months for G2 tumors, and 30.67±6.51 months for G1 tumors. Multivariate analyses revealed that an unknown primary tumor site (p=0.001) and higher histological grade (p < 0.001) were independent prognostic indicators for shorter overall survival (OS). Most long-term survivors (OS > 24 months) had received antitumor treatment.
IntroductionGastroenteropancreatic (GEP) neuroendocrine tumors (NETs) are slowly growing tumors with an indolent course. Most GEP NETs are non-functioning tumors with symptoms related to mass effects or distant metastases [1]. Vague clinical symptoms often delay diagnosis of NETs and distant metastases are commonly detected at the time of initial tumor diagnosis. Apart from regional lymph nodes, the liver is the most frequent site of GEP NET metastases, and hepatic metastases are the most powerful predictor of survival in patients with GEP NET [2]. Up to 75% of patients with small bowel NET and 30%-85% of patients with tumors localized within the pancreas present with liver metastases (LM) either at initial evaluation or during the course of their disease [3-5]. About 20%-50% of GEP NET patients present with LM with unknown primary tumor site [1]. However, most studies incorporating large series of neuroendocrine tumor liver metastases (NETLM) have been performed in Western countries [3,6,7], not in Korea.
The prognosis and clinical management of GEP NETs depend on histological grade. The 2010 World Health Organization (WHO) classification defines the entire group of tumors as neuroendocrine neoplasms and divides the tumors into NET G1, NET G2, and poorly differentiated neuroendocrine carcinoma (G3) based on mitotic count and Ki-67 index [8]. The definition of each grade is as follows: (1) G1: mitotic count, < 2/10 high power fields (HPFs) and/or ≤ 2% Ki-67 index; (2) G2: mitotic count 2-20/10 HPFs and/or 3%-20% Ki-67 index; (3) G3: mitotic count > 20/10 HPFs and/or > 20% Ki-67 index [9]. Surgery is the only curative treatment for GEP NETs [10]. Although NETs generally have a better prognosis than adenocarcinomas at the same site, NETs are incurable once they advance to unresectable metastatic disease.
Various treatment options are available for NETLM [11,12]. Locoregional therapies include surgical resection, radiofrequency thermal ablation (RFA) and transarterial embolization (TAE)/transarterial chemoembolization (TACE) [13]. Locoregional therapies are effective in patients without extrahepatic or synchronous disease. Systemic therapies consist of cytotoxic chemotherapy, somatostatin analogs, vascular endothelial growth factor (VEGF) pathway inhibitors and mammalian target of rapamycin (mTOR) inhibitors [14]. Somatostatin analogs, such as octreotide, can be used to reduce clinical symptoms related to hypersecretion of peptides and amines from tumor cells. Other therapeutic options, such as growth factor pathway inhibitors or mTOR inhibitors, have demonstrated preliminary efficacy [4,14].
Racial and ethnic disparities in cancer outcomes persist [15]; however, most studies on NETLM have been carried out in Western countries. In this study, we examined the clinicopathologic characteristics, primary tumor sites, and prognostic factors in GEP NETLM among Korean patients.
Materials and MethodsWe identified 112 Korean patients with the histological diagnosis of NETLM in the Samsung Medical Center between January 1999 and May 2013. Clinical, radiographic, procedural and pathologic data were extracted from electronic medical records for all 112 patients. Among those, 72 patients with GEP NETLM were enrolled for the present study. The reasons for exclusions (n=40) included primary lung tumors (n=35), primary uterine cervix tumors (n=2) and GEP NETs with limited tissue samples for grading of tumors (n=3).
The formalin-fixed, paraffin-embedded tissues from resected (n=4) or biopsied (n=68) specimens were cut into 3-µm sections and mounted on slides. The slides were deparaffinized for hematoxylin and eosin (H&E) staining. With H&E slides, mitoses were counted per 10 HPFs in the mitotically active region (Fig. 1). In all but three cases, immunohistochemistry for synaptophysin, chromogranin and Ki-67 was performed. The tumors were graded as G1, G2, or G3 following the WHO classification [8].
Macroscopic patterns of liver infiltration by metastases were procured from review of medical records and radiological findings. LMs confined to one liver lobe or limited to two adjacent segments were classified as ‘simple pattern.’ LMs with complex or diffuse patterns were classified as ‘complex pattern’ [13].
Overall survival (OS) was calculated from the date of histological diagnosis of GEP NETLM to the date of the last visit or death. The Cox proportional hazards model was used to determine the variables associated with OS of GEP NETLM. The following variables were modeled: gender (male vs. female), age (< 60 years vs. ≥ 60 years), LM pattern (simple vs. complex), primary site (known vs. unknown), histologic grade (WHO G1+2 vs. WHO G3), and treatment (yes vs. no). Risks are represented as hazard ratios (HR) with 95% confidence intervals (CI). Multivariate survival analyses were also performed to rule out dependent variables. The Kaplan-Meier method with log-rank test was used to compare survival curves divided by influential factors. Null hypotheses of no difference were rejected if p-values were less than 0.05, or, equivalently, if the 95% CIs of risk point estimates excluded 1. All statistical analyses were performed with IBM SPSS Statistics ver. 20 (IBM Co., Armonk, NY).
Results1. Clinicopathological characteristics
Table 1 shows the clinical and pathological characteristics of the 72 patients with GEP NETLM. The median age was 58.94 years (range, 18 to 82 years), and over half of the patients (58%) were men. Twenty-five patients (35%) in our series had NETLM of pancreatic origin, eight patients (11%) had NETLM that originated in the stomach, four patients (6%) had NETLM that originated in the gallbladder, and three patients (4%) had NELM that originated in the rectum. Twenty-five patients (35%) had occult primary tumor and in 20 cases, tissue samples were available for additional immunohistochemistry for TTF1 and CDX2. As expected, all 20 NETLM cases of unknown primary site were negative for TTF1 or CDX2.
In eight patients, LM developed after surgery on the primary GEP NETs. Of the LM patterns, 15 (21%) were simple and 57 (79%) were complex. Twelve patients (17%) had histological grade G1 tumors, 30 patients (42%) had G2 tumors, and 30 patients (42%) had G3 tumors.
Different therapies were administered to the patients. Locoregional therapies included surgical resection, RFA, and TAE/TACE. Systemic therapies included cytotoxic chemotherapy, somatostatin analogs (octreotide), VEGF pathway inhibitors, and mTOR inhibitors. Overall, 37 patients (51%) received chemotherapy alone and 28 patients (39%) did not receive any antitumor treatment. Some patients were treated with combined therapeutic options; patients grouped by management are displayed in Table 2.
The median observation period was 18.01 months (range, 1 to 96 months). The mean follow-up period (OS) was 30.67±6.51 months for histological grade G1 tumors, 19.67±4.09 for G2 tumors, and 11.30±2.44 for G3 tumors. The best OS was recorded for patients with NETLM of pancreatic origin (25.56±5.51 months). Patients with occult primary tumor exhibited poor OS (9.72±2.33 months).
2. Survival analysisUnivariate analyses using a Cox regression model revealed that unknown primary tumor (p=0.005), higher histological grade (p=0.002), and no treatment (p=0.003) were significantly correlated with shorter OS (Table 3). Kaplan-Meier survival curves also demonstrated that the unknown primary tumor site (p=0.003), higher histological grade (p=0.001), and no treatment group (p=0.002) had significantly shorter OS (Figs. 2-4). Multivariate analyses showed that an unknown primary tumor site (HR, 2.954; 95% CI, 1.589 to 5.493), higher histological grade (HR, 3.385; 95% CI, 1.861 to 6.158), and no treatment (HR, 2.332; 95% CI, 1.285 to 4.231) were independent prognostic indicators for shorter OS (Table 3). After exclusion of patients with cachexia (n=8), treatment itself was not significantly associated with OS (HR, 1.837; 95% CI, 0.964 to 3.500). Sixteen patients were long-term survivors (OS > 24 months) and all but one received antitumor treatment. The untreated long-term survivor was a 52-year-old woman. The OS of this patient was 71 months. The patient had primary pancreatic NETs and low mitotic count (1/10 HPFs) in the tumors.
To avoid the possible confounding effects of “unknown” primary tumor sites, we excluded NETLM cases with unknown primary site (n=25) and analyzed 47 GEP NETLM with known primary sites. Univariate analyses using a Cox regression model showed that higher histological grade (p=0.029) and no treatment (p=0.028) were significantly associated with shorter OS (Appendix 1). However, patients’ gender, age or LM pattern were not associated with OS (p > 0.05). Higher histological grade (HR, 2.275; 95% CI, 1.122 to 4.614; p=0.023) and no treatment (HR, 2.433; 95% CI, 1.138 to 5.200; p=0.022) remained significant in multivariate analyses (Appendices 2 and 3). However, after exclusion of patients suffering from cachexia (n=3), treatment itself was not an independent prognostic factor (HR, 1.807; 95% CI, 0.793 to 4.115; p=0.159).
DiscussionThis study analyzed primary sites and factors affecting prognosis in GEP NETLM among Korean patients. The most common primary sites were the pancreas (35%), stomach (11%) and gall bladder (6%). The origin remained unknown in 35% of cases. Unknown primary tumor and higher histological grade were independent prognostic factors in GEP NETLM. The survival rates for NET disease differ according to the anatomical tumor location, being the worst for pancreatic tumors and more favorable for tumors arising in the appendix and localized rectal NETs [3]. In 12.9% of NET patients, metastases were already detectable at the time of initial tumor diagnosis [3]. Apart from the regional lymph nodes, the liver was the predominant site of NET metastases [5]. In a report by Wang et al. [16] out of 123 NETLM patients in the United States, the common primary tumor sites were the pancreas (35.0%), small intestine (26.8%), colon/rectum (12.2%), lung (4.1%), thyroid (1.6%), and stomach (0.8%). They found that 22 cases (17.9%) were of unknown origin, and the primary site was confirmed as small intestine in 13 of 15 patients with unknown primary after surgical exploration. Given this finding, pancreas and small intestine are both common primary tumor sites in NETLM patients in the United States. In the current study of 72 Korean GEP NETLM patients, the most frequent primary tumor site was the pancreas (35%) followed by the stomach (11%), gall bladder (6%), rectum (4%), colon (3%), duodenum (3%), ampulla of Vater (3%), and bile duct (1%). Hence, the most frequent primary tumor site was the pancreas in both American and Korean patients. In the United States, the incidence rate of primary tumors of the small intestine was considerably higher than that in Korea. In contrast to the low rate in the USA, the stomach was the second most common primary tumor site in Korea. The reason for the relatively high rate for the stomach and relatively low rate for the small intestine in Korea is unknown. In a recent Korean multicenter study on distribution of 4,951 GEP NETs according to the organ system, rectum was the most frequent diagnosis, followed by stomach and pancreas, suggesting gastric NET is not uncommon among Koreans [17]. Those distributions would help clinicians explore primary tumor sites during management of patients with GEP NETLM.
In this study, unknown primary tumor was an independent unfavorable prognostic factor for OS. Wang et al. [16] reported that for patients with NETLM and unknown primary tumor, surgical exploration effectively identified and resected occult primary tumors that were often located in the small intestine. These results suggest that, for patients with NETLM and unknown primary tumor, localization and resection of the primary tumor should be considered to treat and prevent complications (i.e., pain, obstruction, ischemia, or perforation) and, perhaps, improve survival.
The 5-year survival rate for metastatic gastrointestinal NET patients undergoing surgical resection is reported to be 47% to 82% [18]. Surgical resection is especially effective in controlling symptoms and improving the quality of life. The surgical approach for NETLM was beneficial with regard to OS compared with the nonsurgical approach; however, curative surgery is only applicable in 10% of the patients [19]. RFA provides prompt symptomatic relief. Embolization of the hepatic artery is effective in palliative management. Chemotherapy is used in patients with highly proliferative NETs such as endocrine pancreatic tumors. Somatostatin analogs, including octreotide, are used effectively subcutaneously, improving symptoms related to the hypersecretion of peptides and amines from tumor cells [12]. The prognosis remains dismal if no treatment is offered [20]. In the present study, those patients who did not receive any antitumor treatment had a shorter OS compared to treated patients, and most long-term survivors received antitumor treatment, thus confirming that the clinical behavior of NETLM was improved by antitumor treatment.
NETs have long been thought to be mostly benign. However, these neoplasms often exhibit a malignant clinical course [3]. Yao et al. [7] reported that, in multivariate analysis of patients with well-differentiated to moderately-differentiated NETs, disease stage, primary tumor site and histological grade were predictors of OS (p < 0.001). A recent study showed that all G1 and G2 NETs were associated with the N0/N1 stage, while 27% of G3 NETs were associated with the N2/N3 stage and these differences were statistically significant [21]. In this study of NETLM, 17% were histological grade G1 tumors, 40% had G2 tumors and 43% had G3 tumors. In a recent Korean multicenter study with 4,951 patients with GEP NET, G1 consisted 92.3%, G2 in 4.85% and G3 in 2.84% [17]. However, in a study with pancreatic NETLM [5], G2 tumors (60.1%) were the most frequent grade followed by G3 (20.2%) and G1 (19.7%) tumors and the proportion of G1 in NETLM was similar to the present study. Histological grade is a major prognostic factor for gastrointestinal NETs [22]. In this study, histological grade G3 was an independent unfavorable prognostic factor for NETLM, consistent with a recent report by Panzuto et al. [23], who found that the major risk factor for progression of metastatic and locally advanced pancreatic endocrine carcinomas was histological grade.
To our knowledge, this study represents the first comparison of primary tumor sites in GEP NETLM in Western countries with those in Korea. The most frequent primary tumor site in GEP NETLM was the pancreas in both the United States and Korea. Unknown primary tumor, higher histological grade and no treatment were independent prognostic indicators for shorter OS. Patients identified as being at a risk of shorter OS should be observed closely.
AcknowledgmentsThis study was supported by a grant of the Korea Healthcare Technology R&D project, Ministry for Health & Welfare Affairs, Republic of Korea, NRF-2012R1A1A3015504 and Samsung Biomedical Research Institute (SBRI-SMX1132721).
Fig. 1.Representative microscopic findings of grade 1 (A), grade 2 (B), and grade 3 (C) neuroendocrine tumors metastasized to the liver (H&E staining, ×40). 
Fig. 2.Kaplan-Meier overall survival stratified by primary sites. Mean overall survival for patients with known primary sites was 22.43±3.25 months compared to 9.72±2.33 months for patients with unknown primary sites (p=0.003). 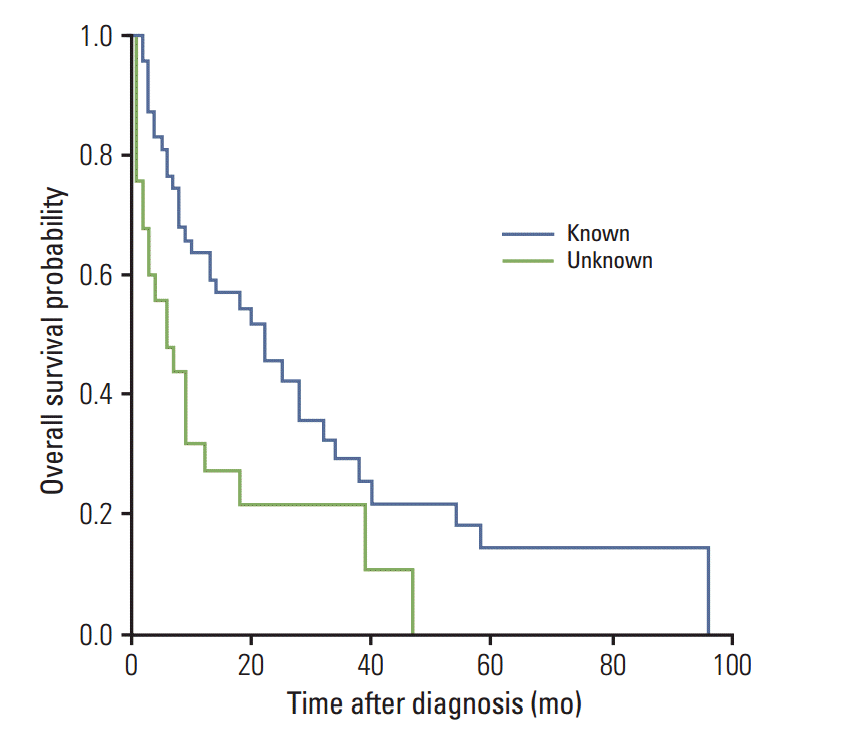
Fig. 3.Kaplan-Meier overall survival stratified by World Health Organization (WHO) grades. Mean overall survival for patients with WHO G1 and G2 tumors was 22.81±3.51 months compared to 11.30±2.44 months for patients with G3 tumors (p=0.001). 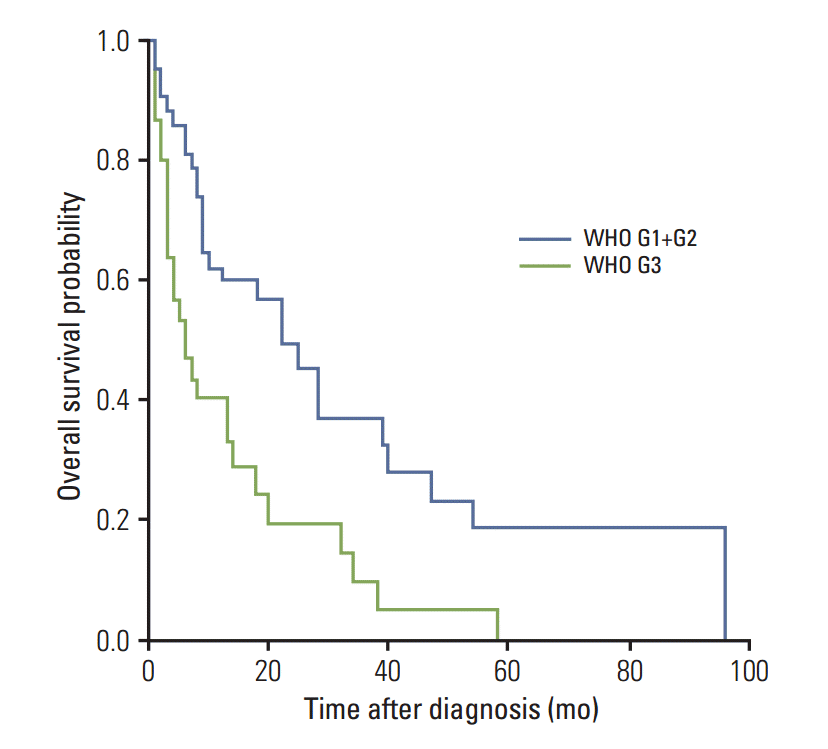
Fig. 4.Kaplan-Meier overall survival stratified by treatment. Mean overall survival for patients with any treatment was 23.59±3.27 months compared to 9.25±2.57 months for patients without treatment (p=0.002). 
Table 1.Clinicopathological characteristics of 72 gastroenteropancreatic neuroendocrine tumors with hepatic metastases Table 2.Treatments used for gastroenteropancreatic neuroendocrine tumors with liver metastases Table 3.Overall survival in 72 patients References1. Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72.
2. Rindi G, D'Adda T, Froio E, Fellegara G, Bordi C. Prognostic factors in gastrointestinal endocrine tumors. Endocr Pathol. 2007;18:145–9.
3. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59.
4. Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25:458–511.
5. Frilling A, Sotiropoulos GC, Li J, Kornasiewicz O, Plockinger U. Multimodal management of neuroendocrine liver metastases. HPB (Oxford). 2010;12:361–79.
6. Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113:2655–64.
7. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
8. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. World Health Organization classification of tumours, No. 3. Lyon: IARC Press; 2010.
9. Rindi G, de Herder WW, O'Toole D, Wiedenmann B. Consensus guidelines for the management of patients with digestive neuroendocrine tumors: the second event and some final considerations. Neuroendocrinology. 2008;87:5–7.
10. Janson ET, Sorbye H, Welin S, Federspiel B, Gronbaek H, Hellman P, et al. Nordic Guidelines 2010 for diagnosis and treatment of gastroenteropancreatic neuroendocrine tumours. Acta Oncol. 2010;49:740–56.
11. Sutcliffe R, Maguire D, Ramage J, Rela M, Heaton N. Management of neuroendocrine liver metastases. Am J Surg. 2004;187:39–46.
12. Granberg D, de Herder W, O'Toole D, Kvols L. Treatment of liver metastases in patients with neuroendocrine tumors. Int J Hepatol. 2012;2012:790635.
13. Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmuller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–76.
14. Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23.
15. Printz C. Racial and ethnic disparities in breast cancer: experts gain new clues about differences in mortality rates among racial groups. Cancer. 2013;119:3739–41.
16. Wang SC, Parekh JR, Zuraek MB, Venook AP, Bergsland EK, Warren RS, et al. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Arch Surg. 2010;145:276–80.
17. Gastrointestinal Pathology Study Group of Korean Society of Pathologists, Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, et al. Current trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea 2000-2009: multicenter study. Cancer Res Treat. 2012;44:157–65.
18. Hodul P, Malafa M, Choi J, Kvols L. The role of cytoreductive hepatic surgery as an adjunct to the management of metastatic neuroendocrine carcinomas. Cancer Control. 2006;13:61–71.
19. Mayo SC, de Jong MC, Bloomston M, Pulitano C, Clary BM, Reddy SK, et al. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol. 2011;18:3657–65.
20. Cheung TT, Chok KS, Chan AC, Tsang S, Dai JW, Lang BH, et al. Long term survival analysis of hepatectomy for neuroendocrine tumour liver metastases. ScientificWorldJournal. 2014;2014:524045.
21. Salama A, Badawy O, Mokhtar N. Ki-67 is a powerful tool for grading neuroendocrine tumors among Egyptian patients: a 10-year experience. J Cancer Res Clin Oncol. 2014;140:653–61.
AppendicesAppendix 1.Overall survival in 47 patients with known primary tumor site |
|
|||||||||||||||||||||||||||||||||||||||||||