AbstractPurposeThe aim of this study is to identify the prognostic factors of distant metastasis (DM) after induction chemotherapy (IC) followed by concurrent chemoradiotherapy (CRT) for locoregionally advanced head and neck cancer (HNC).
Materials and MethodsA total of 321 patients with HNC who underwent IC followed by CRT treated between January 2005 and December 2010 were analyzed retrospectively. IC consisted of three courses of docetaxel (70 mg/m2) and cisplatin (75 mg/m2) every three weeks, followed by radiotherapy of 66-70 Gy/2 Gy per fraction/5 fractions per week concurrent with weekly cisplatin (40 mg/m2). Tumor/nodal stage, primary site, tumor differentiation, lower neck node involvement (level IV, VB, and supraclavicular regions), number of concurrent chemotherapy cycles, overall duration of radiotherapy, and response to IC were assessed as potential prognostic factors influencing DM and survival outcome.
ResultsThe five-year loco-regional recurrence and DM rates were 23.6% and 18.2%. N stage, overall duration of radiotherapy, lower neck node involvement, and response to IC were significant factors for DM. With a median follow-up period of 52 months (range, 4 to 83 months), the 5-year progression-free, DM-free, and overall survival rates were 41.2%, 50.7%, and 55.1%, respectively. Lower neck node involvement (p=0.008) and poor response to IC (p < 0.001) showed an association with significantly inferior DM-free survival.
IntroductionApproximately 60% of patients with head and neck cancer (HNC) present with locoregionally advanced stage III and IV disease [1]. Multiple clinical trials have confirmed the locoregional control and overall survival (OS) benefit of concurrent chemoradiotherapy (CRT), and CRT is now considered the standard management paradigm for locoregionally advanced HNC [2]. The concept of induction chemotherapy (IC) followed by CRT has several theoretical advantages, including reduced risk of distant metastasis (DM), induction of tumor shrinkage to allow more effective and less toxic local therapy, and prediction of tumor responsiveness [3].
Studies over the past two decades have reported DM rates between 4.0% and 26.0% in patients treated for squamous cell carcinoma of the head and neck [4,5]. Preliminary results from a randomized trial (DeCIDE) showed that the incidence of DM at three years was 10% with IC followed by CRT and 19% with CRT alone (p=0.025) [6]. As DM develops, the chance of cure is very low, and the survival decreases dramatically. Some reports have demonstrated an association of locoregional control and risk factors including primary site, nodal stage, tumor differentiation, and lower neck involvement (level IV, VB, and supraclavicular regions) with DM in patients treated with radiotherapy alone or CRT [7,8].
Previous studies analyzed the prognostic factors for survival outcome after treatment with IC followed by CRT [9,10]. However, none of these studies determined the prognostic factors for DM in HNC patients treated with IC and CRT. The incidence and related risk factors of DM in patients treated with IC followed by CRT should be precisely assessed. The objective of this study was to re-evaluate prognostic factors known as risk factors for DM in patients treated with CRT for HNC, and to determine whether or not these factors still have an effect on DM and survival after addition of IC to CRT.
Materials and Methods1. PatientsOf 355 patients treated with IC and CRT for HNC in two institutions between January 2005 and December 2010, data for 321 consecutive patients were reviewed in this retrospective study. Fourteen patients who presented with disease progression after receiving a second course of IC, 12 patients who underwent salvage neck dissection prior to CRT, and eight patients who refused further therapy were excluded from this study. All patients were diagnosed with previously untreated, biopsy-proven squamous cell carcinoma. DM was undetected from computed tomography (CT) or positron emission tomography (PET)-CT scans at the time of their initial diagnosis. Five patients showed N3 stages, but none of them were included in the analysis due to incomplete treatment. All patients showed a performance status of 0 to 1. Patients with primary tumors of the nasopharynx, paranasal sinus, or salivary gland were excluded from this study.
Clinically significant lymph nodes in the cervical region were distinguished according to the criteria described by van den Brekel et al. [11] (i.e., shortest axis of ≥ 11 mm in the jugulodigastric regions, or ≥ 10 mm in other cervical regions). In addition, conglomerate lymph nodes of borderline size on CT, or any positive results for neck lymph nodes from PET scans, were considered metastatic lesions. The TNM stages of the patients were re-evaluated from their medical records and images at the time of data analysis. Patients were staged according to the 2009 classification of the American Joint Committee on Cancer Staging (AJCC) [12]. The study protocol was approved by the institutional review board (IRB).
2. TreatmentIC consisted of three courses of docetaxel (70 mg/m2 as 1-hour infusion) and cisplatin (75 mg/m2 as 2-hour infusion) every three weeks. Three weeks after completion of the second course of IC, response to IC was evaluated using CT scan. If disease progression was noted, the third course of IC was cancelled and surgical treatment was considered. Neck dissection was performed prior to CRT in some cases where multiple necrotic lymph nodes showing a stable response after IC were presented. Radiotherapy was recommended for patients determined as unresectable or who refused surgery.
After completion of IC, CRT was initiated within four weeks. Conventional fractionated radiation therapy with a daily dose of 2 Gy and a total dose of 66-70 Gy was planned for all patients. Concurrent with radiation therapy, administration of six courses of chemotherapy using weekly cisplatin (40 mg/m2 as 1-hour infusion) was planned for patients.
Three-dimensional conformal radiation therapy (3D-CRT) or intensity-modulated radiation therapy (IMRT) was performed on a 6 MV linear accelerator (Clinac 21EX, Varian Medical Systems, Palo Alto, CA). The gross tumor volume (GTV) was defined as the pre-IC gross disease volume (primary and node) shown on imaging studies. The clinical target volume (CTV) encompassed the GTV plus a margin of 0.5-1.0 cm for the potential microscopic extension of the disease, and an additional margin of 0.3 cm was considered to compensate for setup uncertainty. Segmentation of nodal CTV into two parts (CTV2 and CTV3) was performed according to estimation of the risk. Standard doses were 66-70 Gy for CTV1 (the area of primary tumor and metastatic nodes), 60 Gy for CTV2 (CTV1 plus the next echelon nodal area or ipsilateral cervical nodal chains), and 50 Gy for CTV3 (contralateral lymph nodal area or uninvolved lower neck nodal region).
3. Response assessment and follow-upResponse to treatment was documented using the World Health Organization (WHO) response grading system [13]. The response was evaluated between six and 12 weeks after completion of CRT by physical examination and radiological images (CT, magnetic resonance imaging [MRI], and PET-CT). A biopsy was recommended if there was clinical evidence of residual tumor. A complete response (CR) of the primary site was defined by the disappearance of disease evidence on physical examination, radiological images, or pathological reports. In case of neck nodes, CR included all lymph nodes less than 1.0 cm in greatest axial dimension without contrast enhancement on CT scans and with negative results on PET scans. Patients with less than a CR were recommended to go ahead with additional surgery or close observation. Patients were observed by all members of the multidisciplinary team after completion of therapy. Careful clinical examination including imaging studies (CT, ultrasonography and PET, or MRI) was performed at 1-3 month intervals over the first year, every 3-6 months in the second and third years after treatment, and every 6-12 months thereafter.
4. Statistical analysesSurvival times were calculated from the initial date of IC. The Kaplan-Meier method was used for progression-free survival (PFS), distant metastasis-free survival (DMFS), and OS. Except for the OS calculations, a patient was considered censored at death if the event had not occurred. A logistic regression model was used for multivariate analysis of risk factors related to DM. As stated in the Introduction, previous studies reported an association of five variables; tumor stage, nodal stage, primary site, tumor differentiation, and lower neck node involvement with development of DM in patients treated with radiotherapy alone or CRT [7,8]. Therefore, these tumor-related factors were assessed as potential prognostic factors having an effect on DM, PFS, DMFS, and OS. Treatment related factors, including number of concurrent chemotherapy cycles and overall duration of radiotherapy were also evaluated as variables. Response to IC was included in the potential prognostic factors in order to evaluate its predictive role for disease control and survival outcome. We performed multivariate analysis with a logistic regression model and used a binomial distribution statistical model to determine the 95% confidence interval of the probability of development of DM. Multivariate analyses of survival outcome were performed using a Cox’s regression model. PASW software (PASW ver. 18.0, SPSS Inc., Chicago, IL) was used for the statistical analyses. p < 0.05 was used to indicate statistical significance.
Results1. Patient characteristicsThe median age of patients was 60 years (range, 27 to 79 years), and 275 patients (85.7%) were men. Three hundred five patients (95.0%) were treated with IMRT, and 16 patients (5.0%) received 3D-CRT. The median dose (for CTV1) of radiation therapy was 70 Gy (range, 56 to 70 Gy), delivered in a median of 35 fractions (range, 28 to 35 fractions) over 45-73 days (median, 51 days). The tumor characteristics are described in Table 1.
2. Compliance with treatmentThree hundred eleven patients (96.9%) received the prescribed total radiotherapy dose (range, 66 to 70 Gy). In the majority of patients (304 patients, 94.7%), the overall treatment time for radiotherapy was ≤ 8 weeks. Seventeen patients (5.3%) required more than eight weeks to complete treatment because of a variety of treatment-induced toxicities. All patients received three cycles of IC. The dose of docetaxel in the third cycle was reduced by 25% in 21 patients who presented with grade III neutropenia. Two hundred thirty-six patients (73.5%) completed all six cycles of concurrent chemotherapy; however, 23 patients (7.2%) received less than five cycles of weekly cisplatin.
3. Disease controlAccording to results for response to IC, 59 patients showed a CR (18.4%), 208 showed a partial response (PR; 64.8%), and 54 showed an stable disease (SD; 16.8%). Responses after CRT were as follows: CR at the primary site was seen in 227 patients (70.7%); 201 patients (62.6%) had CR of the metastatic lymph nodes in the neck; 96 patients (29.9%) had PR at the primary site or lymph node; treatment failure occurred in 59 of 211 patients (28.0%) who presented with complete remission at the three-month post-treatment assessment; local recurrence was noted in 41 patients, isolated regional recurrence in 13 patients, and locoregional recurrence in nine patients; DM was detected in 24 patients who had achieved CR after CRT—in 18 of these patients, development of DM was preceded by occurrence of locoregional failure; and DM manifested in 36 patients with persistent locoregional disease following treatment. Detailed data regarding the patterns of failure are shown in Fig. 1. The 5-year locoregional recurrence and distant metastasis rates were 23.6% and 18.2%, respectively. At the time of the last follow-up, DM was reported in 60 of 321 patients (18.7%). The sites of metastases were distributed as follows: lung, 28 cases (8.7%); liver, 15 cases (4.7%); bone, 12 cases (3.7%); and multiple sites (lung and bone), five cases (1.6%). Results of multivariate analysis of risk factors related to DM are shown in Table 2. Significantly high risk of DM was observed in patients with N2c stage, lower neck node involvement, prolonged overall duration of radiotherapy, and poor response to IC.
4. Survival outcomeThe median follow-up period for all patients and survivors was 52 months (range, 4 to 83 months) and 68 months (range, 33 to 90 months), respectively. At the time of analysis, 132 patients (41.1%) had died. The causes of death were as follows: cancer progression in 103 patients, other causes in 24 patients, treatment-related toxicity in three patients, and undetermined in two patients. The PFS, DMFS, and OS at five years were 41.2%, 50.7%, and 55.1%, respectively. Various factors related to survival outcomes were evaluated by multivariate analysis, and the results are shown in Tables 3 and 4, respectively.
In multivariate analysis, the significant factors affecting DMFS and PFS were lower neck node involvement (p=0.008 and p= 0.021, respectively) and response to IC (p < 0.001 and p < 0.001, respectively). The DMFS rate at five years according to lower neck lymph node (positive vs. negative) was 34.3% versus 55.2% (Fig. 2) and response to IC (CR, PR, and SD) was 68.9%, 47.3%, and 0% (Fig. 3), respectively. However, OS was not affected by any of these prognostic factors.
DiscussionThe patterns of failure have changed in patients with squamous cell HNC [14,15]. Although CRT programs have provided improved results in locoregional control compared with radiotherapy alone, the failure rate at distant organs remains high. Thus, systemic control of micrometastases has emerged as an important treatment goal. Addition of IC to CRT protocols resulted in a modest reduction in the incidence of DM. In particular, taxane-based IC followed by cisplatin-based CRT is receiving much attention as a new therapeutic approach for treatment of advanced HNC and for organ preservation. The cancer treatment team in our hospital developed a protocol using docetaxel in combination with cisplatin as the IC regimen prior to CRT (weekly cisplatin). 5-Fluorouracil (5-FU) was omitted from the IC regimen because it may induce mucosal toxicity and cumulative myelosuppression. In a previous study, this regimen showed a comparable overall response rate (82.9%) and feasibility with docetaxel, cisplatin, and 5-FU based induction regimen [16]. In our study, of 321 eligible patients, 236 patients (73.5%) completed the full schedules of this program, and overall response rate to IC was 83.2%. In the docetaxel, cisplatin, and 5-fluorouracil (TPF) induction arm of a TAX 324 study, 27% patients did not complete the full schedule, and the overall response rate after IC was 72% [17].
This study demonstrated that lower neck node involvement and response to IC have an effect on the risk of DM and survival outcome despite the addition of aggressive systemic treatment. The 5-year DM rate in our study was 18.2%, slightly higher compared with results from some recent clinical studies on this topic. From the results of some trials, the DM rate varied between 7% and 17% in HNC patients treated with IC followed by CRT [7]. However, in these previous studies, 15-20% of patients were N0 stage. Considering that only 5.3% of patients presented with N0 stage and two-thirds of patients presented with N2b or N2c stage in this study, our results appear to be quite acceptable. Of 60 patients who presented with DM at last follow-up, 36 patients showed persistent locoregional disease after completion of treatment and locoregional recurrence was preceded DM in 18 patients. This means, of course, that locoregional control is also an important predictive factor related to development of DM, except lower neck node involvement or response to IC.
Alvi and Johnson [18] reported an average time from development of DM to death of only five months. As a consequence, identification of groups of patients who are at high risk of developing DM is very important. Risk factors for DM are a matter of debate. Some reports have shown that the risk of DM is greater for nasopharyngeal and hypopharyngeal cancer [5,19]. Conversely, according to other authors [18,20], the site of the tumor had no significant influence on development of DM. In addition, there is disagreement as to the influence of the histologic grade and local extension of the tumor in the appearance of DM [4,5,18]. In our study, tumor related factors (N stage and lower neck node involvement) and treatment related factors (overall duration of radiotherapy and response to IC) showed association with development of DM.
The 5-year rate of distant metastases in patients with lymph nodes confined to the upper neck was reported as 15%, compared with 28% in oropharyngeal cancer patients with lymph nodes in the mid and/or lower jugular chains (p=0.01) treated with radiotherapy alone [21]. The influence of node location on survival periods was analyzed in previous studies. Patients with lower neck node involvement had a worse prognosis because of their tendency for vascular dissemination. The adverse effect of lower neck node involvement was reported by Kalnins et al. [22] in their study of 450 patients who underwent radical neck dissection for oral cavity carcinoma. They clearly demonstrated an inferior survival rate when metastatic adenopathy was found in the lower neck (5-year OS: upper 38%, mid 19%, and lower 14%). Our results showed a marked association of lower neck node involvement with an increased probability of developing DM. Although IC was added prior to CRT to overcome DM, patients with lower neck node involvement still showed a higher rate of DM.
In this study, the PFS, DMFS, and OS at five years were 41.2%, 50.7%, and 55.1%, respectively. These results compare favorably with the outcomes of some trials testing the role of IC. The TAX 323 study reported a 2-year OS rate of 43% in patients treated with induction TPF and radiotherapy [23]. The TAX 324 trial reported a 3-year OS of 62% after TPF followed by radiotherapy concurrent with weekly carboplatin [17]. However, the patient characteristics in our study showed a more advanced stage (in particular, nodal stage ≥ N2, 75.1% vs. 63.0%) than those of patients in the TAX 324 study. Despite these adverse factors, our study presented 5-year OS rates consistent with the survival outcomes of the TAX 324 study. In addition, the locoregional failure rate (23.6%; median follow-up, 52 months) was comparable with that of the TAX 324 study (30%; median follow-up, 42 months).
In the risk factor analysis of the TAX 324 trial, WHO performance status of 1, non-oropharynx site, T3/4 stage, N3 status, and prolonged radiation treatment time showed association with significantly inferior OS [9]. In the current study, lower neck node involvement and response to IC were significant prognostic factors for survival outcome (DMFS and PFS), although they did not show significant association with OS. In univariate analysis, non-oropharyngeal primary sites showed less favorable PFS than orophaynx; however, this was not significant in multivariate analysis. We assume that the failure of systemic control in patients with lower neck node involvement induces poor survival outcome. Investigation of alternative therapeutic approaches may be needed for treatment of patients with metastatic lower neck nodes. A new adjuvant chemotherapy program after completion of CRT or an innovative combination of IC regimen in patients with metastatic lower neck node should be developed in the near future.
Clinical data from only two treatment centers and the retrospective nature of the study design could constitute other pitfalls. However, to the best of our knowledge, this study is the first analysis of DM-related risk factors in patients treated with IC followed by CRT. Considering the purpose of IC, these results are helpful for determining the appropriate treatment strategy for patients with lower neck node involvement and failure to respond after IC.
ConclusionEven with the addition of IC to control DM, the DM rate and survival outcome were poor when metastatic lower neck lymph nodes were present or when patients failed to respond after receiving IC. Investigation of alternative therapeutic approaches may be needed for treatment of patients with metastatic lower neck nodes or who failed to respond after IC.
Fig. 3.Distant metastasis-free survival according to response to IC. IC, induction chemotherapy; CR, complete response; PR, partial response; SD, stable disease. 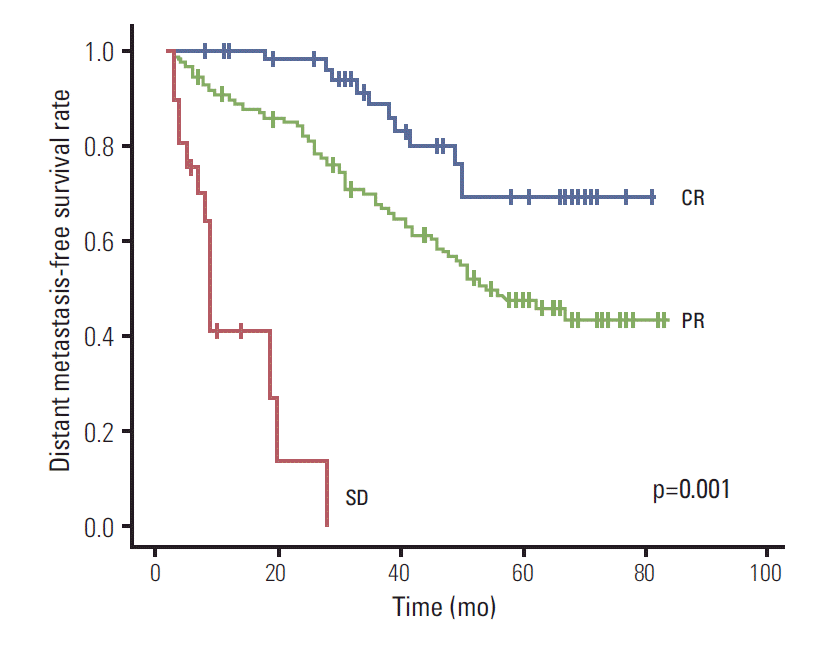
Table 1.Tumor characteristics
Table 2.Multivariate analysis of risk factors for distant metastasis Table 3.Multivariate analysis correlating prognostic factors with 5-year distant metastasis-free survival (DMFS) Table 4.Multivariate analysis correlating prognostic factors with 5-year progression-free survival (PFS) References1. Lee JH, Song JH, Lee SN, Kang JH, Kim MS, Sun DI, et al. Adjuvant postoperative radiotherapy with or without chemotherapy for locally advanced squamous cell carcinoma of the head and neck: the importance of patient selection for the postoperative chemoradiotherapy. Cancer Res Treat. 2013;45:31–9.
2. Adelstein DJ, Li Y, Adams GL, Wagner H Jr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neckcancer. J Clin Oncol. 2003;21:92–8.
4. Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71:452–6.
5. Leon X, Quer M, Orus C, del Prado Venegas M, Lopez M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680–6.
6. Cohen EE, Karrisson T, Kocherginsky M, Huang CH, Agulnik M, Mittal BB, et al. DeCIDE: a phase III randomized trial of docetaxel (D), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol. 2012;30(S):Abstr 5500.
7. Garavello W, Ciardo A, Spreafico R, Gaini RM. Risk factors for distant metastases in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:762–6.
8. Ellis ER, Mendenhall WM, Rao PV, Parsons JT, Spangler AE, Million RR. Does node location affect the incidence of distant metastases in head and neck squamous cell carcinoma? Int J Radiat Oncol Biol Phys. 1989;17:293–7.
9. Sher DJ, Posner MR, Tishler RB, Sarlis NJ, Haddad RI, Holupka EJ, et al. Relationship between radiation treatment time and overall survival after induction chemotherapy for locally advanced head-and-neck carcinoma: a subset analysis of TAX 324. Int J Radiat Oncol Biol Phys. 2011;81:e813–8.
10. Bhide SA, Ahmed M, Barbachano Y, Newbold K, Harrington KJ, Nutting CM. Sequential induction chemotherapy followed by radical chemo-radiation in the treatment of locoregionally advanced head-and-neck cancer. Br J Cancer. 2008;99:57–62.
11. van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177:379–84.
12. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th edNew York: Springer; 2009.
13. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14.
14. Vokes EE, Kies MS, Haraf DJ, Stenson K, List M, Humerickhouse R, et al. Concomitant chemoradiotherapy as primary therapy for locoregionally advanced head and neck cancer. J Clin Oncol. 2000;18:1652–61.
15. Machtay M, Rosenthal DI, Hershock D, Jones H, Williamson S, Greenberg MJ, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20:3964–71.
16. Shin HJ, Chung JS, Choi YJ, Lee BJ, Wang SG, Kim DW, et al. Neoadjuvant docetaxel and cisplatin chemotherapy followed by local irradiation is highly active on locoregionally advanced squamous cell carcinoma of the head and neck. J Laryngol Otol. 2008;122:722–7.
17. Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15.
18. Alvi A, Johnson JT. Development of distant metastasis after treatment of advanced-stage head and neck cancer. Head Neck. 1997;19:500–5.
19. Spector JG, Sessions DG, Haughey BH, Chao KS, Simpson J, El Mofty S, et al. Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. Laryngoscope. 2001;111:1079–87.
20. Johnson JT, Wagner RL, Myers EN. A long-term assessment of adjuvant chemotherapy on outcome of patients with extracapsular spread of cervical metastases from squamous carcinoma of the head and neck. Cancer. 1996;77:181–5.
21. Garden AS, Asper JA, Morrison WH, Schechter NR, Glisson BS, Kies MS, et al. Is concurrent chemoradiation the treatment of choice for all patients with Stage III or IV head and neck carcinoma? Cancer. 2004;100:1171–8.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||