AbstractPurposeTo date, the risk factors for central venous port-related bloodstream infection (CVPBSI) in solid cancer patients have not been fully elucidated. We conducted this study in order to determine the risk factors for CVP-BSI in patients with solid cancer.
Materials and MethodsA total of 1,642 patients with solid cancer received an implantable central venous port for delivery of chemotherapy between October 2008 and December 2011 in a single center. CVP-BSI was diagnosed in 66 patients (4%). We selected a control group of 130 patients, who were individually matched with respect to age, sex, and catheter insertion time.
ResultsCVP-BSI occurred most frequently between September and November (37.9%). The most common pathogen was gram-positive cocci (n=35, 53.0%), followed by fungus (n=14, 21.2%). Multivariate analysis identified monthly catheter-stay as a risk factor for CVP-BSI (p=0.000), however, its risk was lower in primary gastrointestinal cancer than in other cancer (p=0.002). Initial metastatic disease and long catheter-stay were statistically significant factors affecting catheter life span (p=0.005 and p=0.000). Results of multivariate analysis showed that recent transfusion was a risk factor for mortality in patients with CVP-BSI (p=0.047).
ConclusionIn analysis of the results with respect to risk factors, prolonged catheter-stay should be avoided as much as possible. It is necessary to be cautious of CVP-BSI in metastatic solid cancer, especially non-gastrointestinal cancer. In addition, avoidance of unnecessary transfusion is essential in order to reduce the mortality of CVP-BSI. Finally, considering the fact that confounding factors may have affected the results, conduct of a well-designed prospective controlled study is warranted.
IntroductionTotally implantable port systems have been used for more than 20 years for administration of anti-cancer drugs, hydration, and parenteral nutrition. They offer greater advantages over central venous catheters, including their semi-permanent nature, ease of use, and fewer associated complications [1-3]. A further reduction in the complication rate has recently been achieved through the use of ultrasonography to guide insertion [4]. However, several studies have reported a number of common complications after central venous port insertion. Complications include infection (5-26%), thrombosis (2-26%), catheter malposition, catheter fracture, and catheter migration [5,6]. In particular, with respect to infectious complications, special care is needed due to the risk of sepsis related to mortality, the cost of medical treatment, and the duration of hospitalization [7,8]. Therefore, identification of risk factors of central venous port-related bloodstream infection (CVP-BSI) is important for proper management of patients with central venous ports and for adjusting risk in a surveillance program of central venous port in solid cancer [9,10]. To date, the risk factors for CVPBSI in solid cancer patients have not been fully elucidated. In previous studies, incidence and risk for CVP-BSI varied according to the type of cancer [11,12]. In a prospective cohort study, the risk of CVP-BSI was lower in esophageal and colorectal cancer than in other solid cancers, and it was higher for right-sided lines and number of prior line insertions [13]. In another recent study, independent risk factors associated with CVP-BSI were World Health Organization (WHO) performance status between 2 and 4, catheter utilization-days in the previous month, pancreatic cancer, and parenteral nutrition [14].
We conducted this retrospective case control study in order to obtain data from our institution with respect to the incidence of CVP-BSI, the pattern of microbiologic organism, response to antibiotic therapy and risk factors for CVP-BSI in solid cancer patients, because the results of these data differed according to each institution and author.
Materials and Methods1. PatientsWe performed a retrospective review of the medical records of 1,642 patients with solid cancer who underwent central venous port insertion in Seoul St. Mary’s Hospital between October 2008 and December 2011. We evaluated the infectious complications and attempted to determine the primary cause of fever by investigating patients who were considered to have a CVP-BSI based on the result of blood culture test. We defined CVP-BSI as at least one positive blood culture from a peripheral vein and no other apparent source, with positive culture for the catheter segment and a peripheral blood sample; differential period of central venous port culture versus peripheral blood culture positivity of 2 hours; or isolation of the same organism from central and peripheral blood with no other apparent source of infection. These infections were classified according to the definition of catheter related bloodstream infection by the Centers for Disease Control and Prevention (CDC). In the current study, we considered CVP-BSI only as being definite or probable on the basis of above mentioned criteria [15].
2. MethodsThe inserted ports were all implantable catheters, which were made of silicone and the port chamber was composed of titanium. Four types of central venous ports were used: Vortex port (Angio Dynamics Co., UK Ltd., Cambridge, UK), Bard port (Bard Co., Salt Lake City, UT), Vaxcel port (Navilyst Medical Co., Marlborough, MA), and Healthport (Baxter Healthcare SA, Zurich, Switzerland). The insertions were performed in the intervention room of the radiology department. We noted clinical factors including age, sex, diagnosis of primary cancer, underlying disease, the presence of neutropenia or thrombocytopenia, and administration of prophylactic antibiotics.
In this study, we performed a case control study after selection of the control group (non-infection group). In observational studies, the level of causality is usually low because confounding variables are not controlled. Therefore, this study was performed as a case control study by matching the control group using a propensity scoring method in this study in order to increase the level of causality [16].
For patients with CVP-BSI, we selected a control group of patients who were individually matched for age, sex, and insertion time. To determine the risk factors for CVP-BSI, we attempted to determine the existence of statistical differences between the infection group and the matched control group with respect to host, tumor, and catheter factors. The host factors included underlying disease, transfusion after central venous port insertion, and neutropenia occurring after chemotherapy. Tumor factors were primary tumor site, and disease status (localized vs. metastatic), and catheter factors were catheter location (Rt. vs. Lt.) and catheter approach (subclavian vs. jugular). We also investigated monthly catheter-stay duration, which was defined as the number of catheter utilization days per month.
Catheter life span was calculated from the insertion date to the date of death or the removal date. We also determined which of these factors influenced catheter life span, and in the infection group, which factors were associated with death due to CVP-BSI. The survival of each patient was calculated as the duration between the day of CVP-BSI diagnosis and death or the last follow-up day.
We evaluated the statistical significance of the effect of baseline characteristics, microbiology, and the treatment course on the duration of survival. The baseline characteristics included the host, tumor, and catheter factors as described above. Also included was the season in which the infection occurred, the presence of fever, previous surgery history, use of total parenteral nutrition within the previous month, presence of shock, presence of neutropenia and thrombocytopenia, disseminated intravascular coagulation profile, and length of hospital stay. The treatment factors included the timing of antibiotic administration, microbiologic remission, port removal, and presence of combined infection.
3. Statistical analysisAnalysis was performed using SAS ver. 9.2 (SAS Institute Inc., Cary, NC) and SPSS ver. 20.0 (SPSS Inc., Chicago, IL) software packages. We showed descriptive data as a median value (range) and percentage (%). In this study, a propensity scoring method was used for correction of the statistical difference of baseline characteristics between non-randomized groups. We set three variables as matching factors; age, sex, and insertion time, as variables influencing the treatment group. Matching was done for extraction of the control group (non-infection group) matched with the case group (infection- group) according to the matching factors. The insertion date was reported in years and months because the interns or residents who manipulate the central venous ports are routinely changed every month at our hospital. By logistic regression, we calculated the propensity score and performed individual matching according to this score. Finally, we analyzed the matched data. For the 66 patients with CVPBSI, we selected a control group of 130 individually matched patients, and investigated the risk factors for the CVP-BSI group compared to the control group using Cox proportional-hazards regression.
We attempted to determine the factors that influenced catheter life span using the Kaplan-Meier method. In addition, we determined the statistical significance of the effect of baseline characteristics, microbiology status, and the treatment course on the duration of survival by a forward selection procedure using Cox proportional-hazards regression.
Results1. Baseline characteristics of patientsOf 1,642 patients who underwent central venous port insertion, 1,076 patients (65.7%) were male and 562 patients (34.3%) were female, with a combined median age of 63 years (range, 16 to 91 years). The most common cancer was lung cancer in 380 patients (23.1%), followed by upper gastrointestinal tract cancer, including esophageal cancer, stomach cancer in 297 patients (18.1%), colorectal cancer in 236 patients (14.4%), hepatobiliary cancer in 167 patients (10.2%), head and neck cancer in 144 patients (8.8%), and pancreatic cancer in 110 patients (6.7%).
We compared the baseline characteristics of the CVP-BSI and matched control group (Table 1). The median age of patients was 64 years in both groups (range, 23 to 78 years and 25 to 78 years). Thoracic cancer, including lung cancer, and esophageal cancer was more frequent in the infection group (p=0.008), while, on the other hand, gastrointestinal cancer, including stomach cancer, colorectal cancer, and hepatobiliary pancreatic cancer, was more frequent in the noninfection group (p=0.000). In addition to cancer, a number of underlying diseases, including hypertension (21 patients, 32%), diabetes (eight patients, 12%), respiratory disease (five patients, 8%), and cardiovascular disease (three patients, 4.5%) were noted. The subclavian approach was more commonly adopted in the infection group (p=0.045). Transfusion after central venous port insertion was more frequent in the non-infection group (p=0.000).
2. Clinical manifestation and treatment course of the CVPBSI groupThe primary origin of cancer is described in detail (Table 2). CVP-BSI occurred most frequently from September to November (25 patients, 37.9%), and 33 patients (50%) had an infection within one month after chemotherapy (with a median of 21 days after chemotherapy). With respect to tumor response to previous chemotherapy, 26 patients (39.5%) achieved a better than stable disease status, whereas 26 patients (39.5%) experienced progressive disease. After insertion of a chemoport, 19 patients received transfusion before development of CVP-BSI. CVP-BSI showed an association with grade 3 or 4 neutropenia in 11 patients (16.7%) and grade 3 or 4 thrombocytopenia in eight patients (12.1%). Fever was present in 55 patients (83.3%), however, only five patients (7.6%) had accompanying septic shock. After immediate application of antibiotics and removal of chemoport for 64 patients (97%), negative conversion of blood culture test was obtained in 57 patients (86.4%) and death after the occurrence of CVP-BSI was recorded in 17 patients (25.8%). Seven of 17 patients had combined infection that had an influence on mortality. These infections included pneumonia for two patients with lung cancer and one patient with upper gastrointestinal tract cancer, urinary tract infection for one patient with lung cancer and one patient with gynecologic cancer, and gastrointestinal infection for one patient with primary peritoneal cancer and one patient with urogenital cancer.
3. Microorganisms responsible for central venous portrelated infectionsOf 66 patients with CVP-BSI, gram-positive cocci, the most common pathogen, were isolated in 35 patients (53.0%), fungi were isolated in 14 patients (21.2%), gram-negative rod bacteria were isolated in eight patients (12.1%), polymicrobial infection was noted in eight patients (12.1%), and nontuberculosis mycobacteria were isolated in one patient (1.5%) (Table 3). Among the gram-positive cocci infections, methicillin- resistant strains of coagulase negative Staphylococcus and Staphylococcus aureus were isolated in 15 and seven patients, respectively. Streptococcus spp. were present in five patients (7.6%), and Enterococcus spp. in four patients (6.1%). In cases of gram-negative rod infection, seven patients (10.6%) were positive for Pseudomonas spp., three (4.5%) for Klebsiella pneumonia, three (4.5%) for Acinetobacter baumannii, and one (1.5%) for Escherichia coli. The fungal infection rate was relatively higher in our study compared with other reports: there were 10 cases (15.2%) of Candida albicans infection, five cases (7.6%) of Candida glabrata infection, three cases (4.5%) of Candida tropicalis infection, and one case (1.5%) of Candida famata infection.
4. Risk factors for CVP-BSIStatistically significant factors analyzed by univariate analysis included, the presence of primary gastrointestinal cancer and monthly catheter-stay (p=0.002 and p=0.000). By multivariate analysis, monthly catheter-stay was only a statistically significant risk factor for CVP-BSI (p=0.000), however, its risk in primary gastrointestinal cancer was lower than that of other cancers (p=0.002) (Table 4).
5. Factors affecting catheter life spanMedian catheter life span for overall patients was 226 days (range, 6 to 1,104 days). For the CVP-BSI group, median catheter life span was 135 days (range, 6 to 781 days), while in the non-infection group, median catheter life span was 260 days (range, 10 to 1,104 days). Initial localized disease showed longer catheter life span than metastatic disease (p=0.005); and monthly catheter-stay more than seven days showed shorter catheter life span than less than seven days (p=0.000) (Fig. 1).
6. Factors affecting mortality in the CVP-BSI groupWe assessed the question of whether any of the clinical factors were associated with overall mortality resulting from CVP-BSI. In addition to baseline characteristics, we included the monthly catheter utilization days, hospital stay within the last month, disseminated intravascular coagulation profile, duration of admission, use of prophylactic antibiotics, combined infection, and the type of microorganism (grampositive cocci, gram-negative rod, fungus, polymicrobial infection, or specific strains of bacteria). We also considered the treatment course followed by each patient (time to first antibiotic administration, microbiologic remission, central venous port removal, and the presence and type of associated infection). Results of univariate analysis using the Cox regression model indicated that hospital stay within the last month (≥ 1 week), recent transfusion before CVP-BSI and total parenteral nutrition within the last month were all statistically significant factors (p < 0.05) (Table 5). Transfusions were mostly packed red cells by peripheral venous route and performed between admission and occurrence of CVP-BSI. Results of statistical analysis showed that severe neutropenia did not affect the mortality due to CVP-BSI (p=0.299). In the multivariate analysis, recent transfusion was a statistically significant factor for overall mortality of the CVP-BSI group (p=0.013).
DiscussionThe central venous catheter is an important device for delivery of chemotherapeutic drugs, and the central venous port is a totally implantable catheter commonly used because of its convenience, safety, and ability to be implanted for an extended period of time. Many studies have concluded that totally implantable devices had the lowest reported rates of catheter related-bloodstream infection compared to either tunneled or other non-tunneled catheters [17]. However, the risk of infection associated with a central venous port is increased in immunocompromised patients, particularly those with cancer, not only because their catheter is frequently manipulated for treatment, but also due to development of neutropenia after chemotherapy [18].
Results of previous studies on catheter related-bloodstream infections are difficult to interpret because the catheters included central and peripheral or tunneled and non-tunneled catheters [6,19]. However, our study evaluated only totally implantable, non-tunneled central venous ports.
In this study, we set the definition of CVP-BSI as 1) positive tip culture, or 2) differential time to positivity greater than 120 minutes, or 3) isolation of the same organism from central or peripheral blood with no other apparent source of infection. Based on this definition, we identified 66 patients with CVP-BSI from 1,642 solid cancer patients who received a central venous port, and we selected an age-, sex-, and insertion time-matched control group of 130 patients from among those who did not develop an infection.
We did not match the primary origin of cancer because there is no definitive evidence that the primary origin of cancer affects the risk of CVP-BSI [13]. Comparison of the baseline characteristics of the CVP-BSI and matched control groups showed that thoracic cancer was more frequent in the CVP-BSI group, while gastrointestinal cancer was more frequent among the control subjects. Although the subclavian approach was more common in the CVP-BSI group, compared to the control group, only four patients had their central venous port placed via the subclavian approach, while the remaining 62 patients underwent placement via the jugular approach. A higher rate of transfusion after central venous port insertion was observed in the non-infection group compared to the CVP-BSI group. This difference may be because the port was removed in a majority of patients after they developed a CVP-BSI, and, thus, the non-infection group had a relatively longer duration of catheter implantation and therefore more transfusions.
In this study, the most common cancers in patients with CVP-BSI were those of the lung and head and neck.
The frequent CVP-BSI in patients with lung and head and neck cancer could be related to prolonged severe neutropenia induced by high-dose chemotherapy or prolonged radiation therapy. In addition, mucositis of the mouth, nasopharynx, and other respiratory epithelia disrupts the barrier function of the mucosa, leading to microbial invasion [20]. Infections occurred most frequently between September and November, possibly because this season is associated with more cases of influenza and other infectious diseases, so that the immunity of the patients decreased, resulting in development of more cases of CVP-BSI. Similar to recent studies of CVP-BSI associated with neutropenia [21,22], in this study, gram-positive cocci was the most commonly cultured pathogen, accounting for 35 infections (53.0%). Methicillin-resistant strains (coagulase negative Staphylococcus and Staphylococcus aureus) comprised 53% of isolates. This high resistance rate could justify early empirical treatment with glycopeptides for gram-positive central venous port infections. On the other hand, it is noteworthy that Streptococcus species were found in five cases, and these strains may have been associated with mucositis occurring after injury of the oral and intestinal mucosa during severe neutropenic period [23]. The rate of fungal infection was higher than that reported in other studies. Possible explanations for the increased incidence of fungal infection include profound neutropenia after chemotherapy, immunosuppression, frequent administration of total parenteral nutrition, and incorrect manipulation of the central venous port.
In terms of clinical risk factors for CVP-BSI in both univariate and multivariate analyses, monthly catheter-stay duration and primary gastrointestinal cancer were significant. The longer duration of monthly catheter-stay could be related to multiple manipulation of the chemoport, so that the risk of CVP-BSI could be increased. Patients with primary gastrointestinal cancer survived longer and had better performance status than patients with other cancers, such as thoracic cancer; therefore, they had reduced risk of CVP-BSI. This finding is consistent with those of previous prospective studies in which patients with gastrointestinal cancer had lower CVP-BSI risk than patients with other solid tumors [13], and the incidence of CVP-BSI in patients with colorectal cancer was lower than that in patients with other digestive tract cancers [14].
Factors associated with catheter life span were disease status at the time of chemoport insertion, and monthly catheter-stay; similar to the risk factors for CVP-BSI. This similarity in associated factors reflects the fact that most patients with CVP-BSI had to have their central venous port removed. There was a higher rate of infection with methicillin-resistant Staphylococcus aureus, fungi, gram-negative rods, and non-tuberculosis mycobacteria in our study, compared to previous studies. The fact that catheter removal is usually indicated for infections with these organisms explains the higher rate of removal. Early disease progression to death probably had effect on the decrease of catheter life span in metastatic disease compared to limited disease.
We investigated the reason for the higher in-hospital mortality after occurrence of CVP-BSI (17 patients, 25.8%) compared to previous studies and found that CVP-BSI could be considered the true cause of death for only eight patients (12%). We also identified a number of risk factors for mortality in patients with CVP-BSI, including hospital stay within the last month (at least one week), transfusion between admission and occurrence of CVP-BSI, and infusion of total parenteral nutrition within the last month.
The high mortality after a recent transfusion could be explained by several reasons. First, if a transfusion precedes CVP-BSI, the blood transfusion could result in decreased immune function, making the patient vulnerable to CVP-BSI. Several reports have indicated that patients receiving an allogeneic blood transfusion were at increased risk of any type of infection, pointing to possible transfusion-induced immunomodulation [24,25]. Second, CVP-BSI could precede the transfusion and remain asymptomatic until progression to disseminated intravascular coagulation (DIC), ultimately resulting in severe anemia requiring a transfusion. Third, anemia and CVP-BSI could occur simultaneously as a complication of bone marrow suppression after intensive chemotherapy. Therefore, care should be taken in interpreting our results because several factors, including disease status, infection pattern, and associated infections could act as confounding factors.
The risk associated with the recent administration of total parenteral nutrition may be related to the consequent increase in central venous port manipulation, leading to increased opportunity for inoculation with potentially infectious organisms. In other studies, parenteral nutrition was found to be an independent risk factor associated with CVPBSI [14]. Although results of multivariate analysis did not show that parenteral nutrition was statistically significant, we cautiously recommend avoiding systematic infusion of parenteral nutrition.
This study had several limitations. First, it was retrospective and conducted at a single center. Second, the catheter as a possible source of CVP-BSI was investigated only in patients who had blood cultures drawn from both the chemoport and a peripheral vein, thus, the number of patients with true CVP-BSI could have been underestimated. Third, because we did not match the non-infection group by primary origin of cancer, selection bias could have influenced the prevalence results.
ConclusionDespite the safety and convenience of the central venous port, there is a continuing risk of infectious complications. Considering our results for microorganisms, prompt administration of antibiotics covering gram-positive cocci is warranted for patients suspicious of CVP-BSI. If the fever does not resolve within a few days of initial empirical administration of antibacterial agents, antifungal therapy should be considered due to increased incidence of fungal infection. In addition, if the presence of a pathogen which is indicated for catheter removal is confirmed, timely catheter removal is necessary. In analysis of the results with respect to risk factors, prolonged catheter-stay should be avoided as much as possible. It is necessary to be cautious of CVP-BSI in metastatic solid cancer, especially non-gastrointestinal cancer. In addition, avoidance of unnecessary transfusion is essential because it contributes to the mortality of CVP-BSI. Finally, considering the fact that confounding factors may have affected the results, conduct of a prospective controlled study is warranted.
Fig. 1.Factors, initial disease status (A) and monthly catheter-stay duration (B), affecting on catheter life span by Kaplan-Meier analysis. 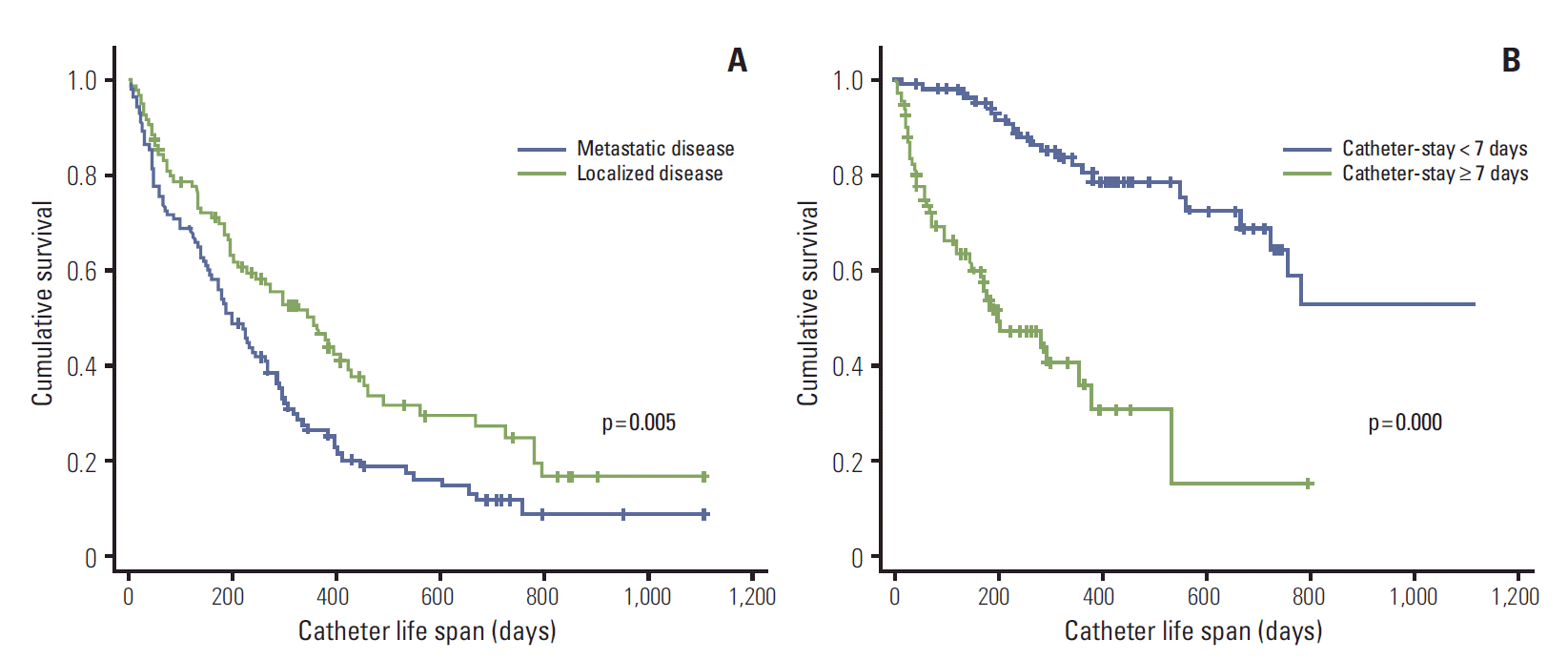
Table 1.Comparisons between the CVP-BSI group and the matched non-infection group
Table 2.Clinical characteristics and outcomes of the CVP-BSI group
Table 3.Clinical characteristics and outcomes of the CVP-BSI group Table 4.Risk factors for CVP-BSI
Table 5.Factors affecting overall in-hospital mortality of the CVP-BSI group
References1. Gyves JW, Ensminger WD, Niederhuber JE, Dent T, Walker S, Gilbertson S, et al. A totally implanted injection port system for blood sampling and chemotherapy administration. JAMA. 1984;251:2538–41.
2. Wolosker N, Yazbek G, Nishinari K, Malavolta LC, Munia MA, Langer M, et al. Totally implantable venous catheters for chemotherapy: experience in 500 patients. Sao Paulo Med J. 2004;122:147–51.
3. Ng F, Mastoroudes H, Paul E, Davies N, Tibballs J, Hochhauser D, et al. A comparison of Hickman line- and Port-a-Cath-associated complications in patients with solid tumours undergoing chemotherapy. Clin Oncol (R Coll Radiol). 2007;19:551–6.
4. Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin. 2008;58:323–46.
5. Kim JT, Oh TY, Chang WH, Jeong YK. Clinical review and analysis of complications of totally implantable venous access devices for chemotherapy. Med Oncol. 2012;29:1361–4.
6. Cortelezzia A, Fracchiolla NS, Maisonneuve P, Moia M, Luchesini C, Ranzi ML, et al. Central venous catheter-related complications in patients with hematological malignancies: a retrospective analysis of risk factors and prophylactic measures. Leuk Lymphoma. 2003;44:1495–501.
7. Raad I, Hachem R, Hanna H, Bahna P, Chatzinikolaou I, Fang X, et al. Sources and outcome of bloodstream infections in cancer patients: the role of central venous catheters. Eur J Clin Microbiol Infect Dis. 2007;26:549–56.
8. Tacconelli E, Smith G, Hieke K, Lafuma A, Bastide P. Epidemiology, medical outcomes and costs of catheter-related bloodstream infections in intensive care units of four European countries: literature- and registry-based estimates. J Hosp Infect. 2009;72:97–103.
9. Tong EN, Clements AC, Haynes MA, Jones MA, Morton AP, Whitby M. Improved hospital-level risk adjustment for surveillance of healthcare-associated bloodstream infections: a retrospective cohort study. BMC Infect Dis. 2009;9:145.
10. Worth LJ, Slavin MA, Brown GV, Black J. Catheter-related bloodstream infections in hematology: time for standardized surveillance? Cancer. 2007;109:1215–26.
11. Groeger JS, Lucas AB, Thaler HT, Friedlander-Klar H, Brown AE, Kiehn TE, et al. Infectious morbidity associated with longterm use of venous access devices in patients with cancer. Ann Intern Med. 1993;119:1168–74.
12. Hsieh CC, Weng HH, Huang WS, Wang WK, Kao CL, Lu MS, et al. Analysis of risk factors for central venous port failure in cancer patients. World J Gastroenterol. 2009;15:4709–14.
13. Mollee P, Jones M, Stackelroth J, van Kuilenburg R, Joubert W, Faoagali J, et al. Catheter-associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78:26–30.
14. Toure A, Vanhems P, Lombard-Bohas C, Cassier P, Pere-Verge D, Souquet JC, et al. Totally implantable central venous access port infections in patients with digestive cancer: incidence and risk factors. Am J Infect Control. 2012;40:935–9.
15. Tomlinson D, Mermel LA, Ethier MC, Matlow A, Gillmeister B, Sung L. Defining bloodstream infections related to central venous catheters in patients with cancer: a systematic review. Clin Infect Dis. 2011;53:697–710.
16. Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–63.
17. Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SR, et al. epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp In. 2007;65 Suppl 1:S1–64.
18. Howell PB, Walters PE, Donowitz GR, Farr BM. Risk factors for infection of adult patients with cancer who have tunnelled central venous catheters. Cancer. 1995;75:1367–75.
19. Kim HJ, Yun J, Kim HJ, Kim KH, Kim SH, Lee SC, et al. Safety and effectiveness of central venous catheterization in patients with cancer: prospective observational study. J Korean Med Sci. 2010;25:1748–53.
20. Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colonystimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013;368:1131–9.
21. Dompeling EC, Donnelly JP, Deresinski SC, Feld R, Lane-Allman EF, De Pauw BE. Early identification of neutropenic patients at risk of grampositive bacteraemia and the impact of empirical administration of vancomycin. Eur J Cancer. 1996;32A:1332–9.
23. Blijlevens NM, Donnelly JP, de Pauw MR. Empirical therapy of febrile neutropenic patients with mucositis: challenge of risk-based therapy. Clin Microbiol Infect. 2001;7 Suppl 4:47–52.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||