Introduction
Stromal cell-derived factor-1 (SDF-1) is a chemokine constitutively expressed and produced in bone marrow stromal cells (BMSCs). It induces the migration and homing of hematopoietic stem cells (HSCs) and progenitor cells (HPCs) through signaling via the G protein-coupled receptor CXCR4 (1). Myeloma cells also express CXCR4 (2) and respond to SDF-1, resulting in the trafficking and localization of these cells in the bone marrow (BM) microenvironment (3). SDF-1 alone has minimal or negligible effects on the survival and growth of myeloma cells in vitro (4), but many reports are consistent with the SDF-1/CXCR4 axis being involved in the progression of myeloma. For example, serum levels of SDF-1 are elevated in patients with multiple myeloma (5), and CXCR4 expression increases in extramedullary plasmacytoma, a manifestation of an advanced stage of multiple myeloma (6). BM endothelial cells in multiple myeloma secrete CXC chemokines, including SDF-1, that mediate interactions with myeloma cells (7). Additionally, SDF-1 plays an important role in tumor neoangiogenesis, and blockade of the SDF-1/CXCR4 axis attenuates tumor growth (8). These observations raise the possibility that modulation of the SDF-1/CXCR4 axis could influence the biology of myeloma cells and the disease course.
AMD3100, a small bicyclam molecule, was originally developed as a CXCR4 antagonist that blocked the entry of the HIV virus into T cells. It inhibits the binding of SDF-1 to CXCR4 and induces peripheral mobilization of HSCs and HPCs (9). AMD3100 also enhances the mobilization of HSCs induced by granulocyte colony-stimulating factor (10). AMD3100 induces the segregation of leukemic cells (11) and myeloma cells (12) in the BM microenvironment, which is expected to enhance the chemosensitivity of the cells. Based on these observations, AMD3100 is about to be used clinically (13) for the peripheral mobilization of HSCs in patients with lymphoma and multiple myeloma. However, AMD3100 has been demonstrated to activate a G protein coupled with CXCR4, and thus acts as a partial CXCR4 agonist in vitro (14). Additionally, AMD3100 was shown to exert dual effects in bleomycin-induced lung inflammation in an animal model (15). Thus, it is necessary to address the question of whether AMD3100 functions as a partial agonist for CXCR4 in lymphoma or myeloma cells before it is widely used in a clinical setting. In this study, we explored whether AMD3100 affected the proliferation and survival of myeloma cells in vitro.
Materials and Methods
1. Cells and reagents
BM samples were obtained, with informed consent, from patients with multiple myeloma at the time of diagnosis. CD138+ cells were purified from BM using the MACS system (Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol. The human myeloma cell lines RPMI8226 and U266 were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and cultured in RPMI-1640 medium (Gibco-BRL Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL Life Technologies). Hepatocellular carcinoma cell lines PLC/PRF5 and Hep3B were purchased from ATCC and cultured in RPMI-1640 and Dulbecco's modified Eagle medium (DMEM; Gibco-BRL Life Technologies), respectively, supplemented with 10% FBS. Interleukin-6 (IL-6) and SDF-1 were purchased from R&D Systems (Minneapolis, MN). AMD3100 and pertussis toxin (PTX) were acquired from Sigma Chemical Co. (St. Louis, MO). Another CXCR4 antagonist T140 peptide (H-Arg-Arg-Nal-Cys-Try-Arg-Lys-d Lys-Pro-Tyr-Arg-Cit-Cys-Arg-OH) was purchased from Peptron Inc. (Daejeon, Korea). Signal-blocking agents which included wortmanin, LY294002, AG490, PD98059, rapamycin and SB203580 were obtained from Calbiotech (Spring Valley, CA).
2. Flow cytometry
Cells were incubated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated monoclonal antibodies at 4℃ for 30 minutes and analyzed using a Coulter Elite flow cytometer (Coulter Electronics Ltd., Hialeah, FL) or FACSCanto II flow cytometer (BD PharMingen, San Diego, CA). The monoclonal antibodies used were FITC- or PE-conjugated anti-CD138 (Mi15; BD PharMingen) and PE-conjugated anti-CXCR4 (12G5; BD PharMingen). To detect cytoplasmic CXCR4, the cells were permeabilized with a saponin-based reagent (BD PharMingen) according to manufacturer's instructions and labeled. To detect apoptosis, cells were stained with FITC-conjugated annexin V (BD PharMingen) according to the manufacturer's instructions and analyzed by flow cytometry.
3. Migration assay
For the transmigration experiments, cells (2×105 cells/well) were loaded into the upper chamber of a 24-well Transwell plate containing a 5-µm microporous membrane (Corning-Costar, Cambridge, MA) and were allowed to migrate into the lower chamber containing 100 g/mL SDF-1 for 4 hours. Migrated cells were counted, and the fold-increase in the number of migrated cells compared to that in the control (migration index) was calculated.
4. Cell proliferation assay
The effects of SDF-1, IL-6, AMD3100, T140 and signal-blocking agents on myeloma cell proliferation were measured using a colorimetric assay kit (CCK-8 assay kit; Dojindo Laboratories, Tokyo, Japan) based on the MTT assay, according to the manufacturer's instructions. Briefly, 5×103 cells were incubated in 96-well plates in serum-free X-VIVO medium (BioWhittaker, Walkersville, MA). To detect the effect of AMD3100 on the proliferation of primary CD138+ cells, IL-6 (20 ng/mL) was added to each well. After incubation, 10 µL of CCK-8 solution provided by the manufacturer was added to each well. After 3h incubation at room temperature, the optical density (OD) was measured using a spectrophotometer (Molecular Devices Co., Sunnyvale, CA) and the fold-increase in the OD compared to that of the control (proliferation index) was calculated.
5. Tumor cell colony assay
Myeloma cells (8×103) were plated in triplicate in 35-mm tissue culture dishes (Corning Costar), containing 1 mL of assay medium consisting of X-VIVO medium (BioWhittaker) and 1.2% methyl-cellulose (StemCell Technologies, Vancouver, Canada). After 3 to 15 days of incubation at 37℃ in 5% CO2 in air, the number of colonies, defined as cell clusters consisting of more than five cells, was scored under an inverted microscope.
6. Western blot analysis
Western blotting was used to detect the phosphorylation of signaling molecules. Cells were starved in serum-free medium for 12 hours and then stimulated with cytokines or AMD3100. The cells were collected by centrifugation, washed in phosphate-buffered saline, and lysed by the addition of SDS sample buffer (187.5 mM Tris-HCl [pH 6.8], 6% [w/v] SDS, 100% glycerol, 150 mM DTT, and 0.03% [w/v] bromophenol blue). Equal amounts of protein from each sample were separated by electrophoresis on 10% SDS-polyacrylamide gels and transferred to polyvinylidene fluoride membranes (Amersham Life Science, Arlington Heights, IL). The membranes were blocked for 1 hour in Tris-buffered saline (TBS) containing 5% (w/v) milk and 0.1% Tween20, and then incubated with the primary mouse or rabbit monoclonal antibody (Cell Signaling Technology Inc., Danvers, MA) overnight at 4℃. The blots were washed with TBS containing Tween20, incubated with anti-mouse or anti-rabbit secondary antibody for 2 hours, and developed using West-Zol Plus (iNtRON Biotechnology, Seoul, Korea). The following antibodies were used: anti-phospho-Akt polyclonal antibody (Ser473), anti-Akt polyclonal antibody, anti-phospho-MAPK p44/p42 polyclonal antibody (Thr202, Tyr204), anti-MAPK p44/42 polyclonal antibody, anti-phospho-Stat3 polyclonal antibody (Tyr705), anti-Stat3 polyclonal antibody, anti-phospho-MAPK p38 polyclonal antibody (Thr180, Tyr182) and anti-MAPK p38 polyclonal antibody (all from Cell Signaling Technology).
Results
1. AMD3100 blocks the migration of myeloma cells in response to SDF-1
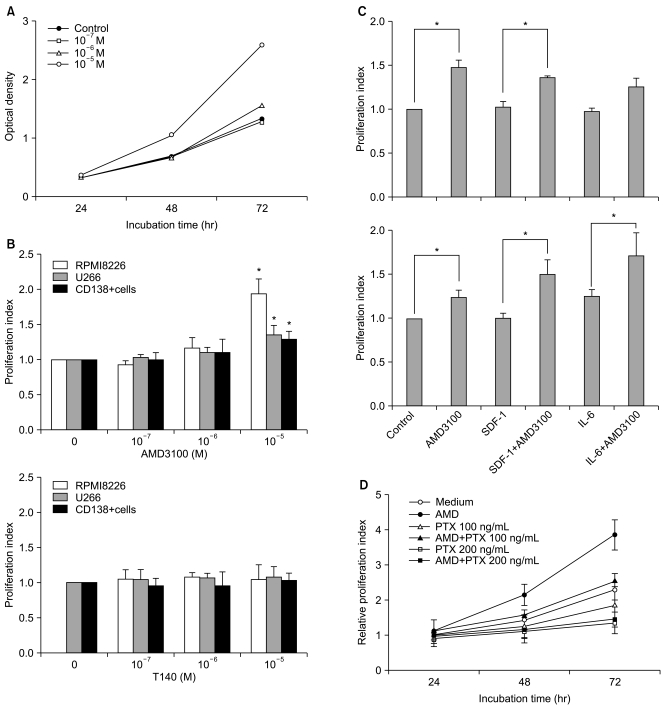
Using flow cytometry, two myeloma cells and primary CD138+ cells were confirmed to express CXCR4 on the cell surface to varying degrees (data not shown). In 4-hour transmigration assays using the Transwell system, SDF-1 in the lower chamber induced the transmigration of myeloma cells and primary BM CD138+ cells, which was abolished by treating the cells in the upper chamber with AMD3100 and T140 (Fig. 1A). Pretreating the cells in the upper chamber with PTX (200 ng/mL) for 2 hours also markedly inhibited the chemotaxis of the cells in response to SDF-1 (data not shown). To further clarify the function of AMD3100 and T140, we examined whether these agents induced the internalization of cell surface CXCR4. Treating U266 cells with 10-5 M AMD3100 or 10-6 M T140 for 3 hours in serum-free X-VIVO medium resulted in the internalization of almost all surface CXCR4 (Fig. 1B).
2. AMD3100, but not T140, stimulates the proliferation of myeloma cells in short-term incubation
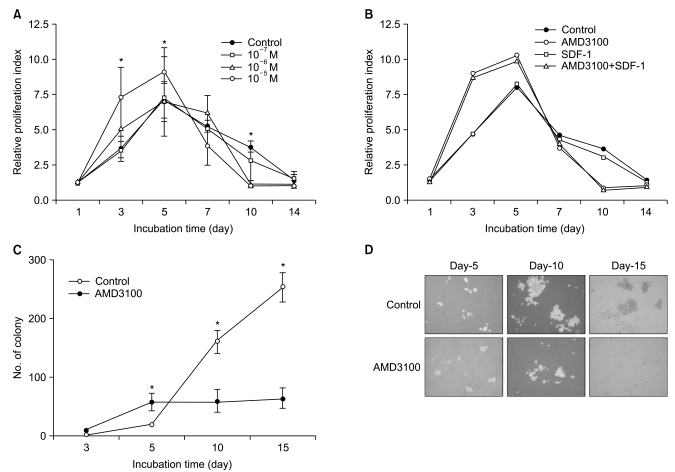
To examine whether SDF-1 and AMD3100 affect the proliferation of myeloma cells, the two myeloma cells and primary CD138+ cells from three patients with multiple myeloma were incubated in serum-free X-VIVO medium in the absence or presence of AMD3100 (10-7 to 10-5 M) or T140 (10-7 M to 10-5 M) for up to 3 days and analyzed by a CCK-8 assay. SDF-1 alone did not affect the proliferation of the myeloma cells (data not shown). AMD3100, but not T140, dose-dependently increased the number of myeloma cells and the primary CD138+ cells. After a 3-day incubation, 10-5 M AMD3100 increased the proliferation of RPMI8226 cells, U266 cells and primary CD138+ cells by 1.8-, 1.4- and 1.3-fold, respectively, compared to the control (all p<0.05; Fig. 2A and 2B). These proliferation-enhancing effects were more prominent in the presence of IL-6 in U266 cells, but not in RPMI8226 cells (Fig. 2C). Pretreating the cells with PTX for 2 hours abrogated the enhancement (Fig. 2D). AMD3100 did not affect the proliferation of hepatocellular carcinoma PLC/PRF5 or Hep3B cells (data not shown), indicating that the proliferation-enhancing effect of AMD3100 is not common to all cells.
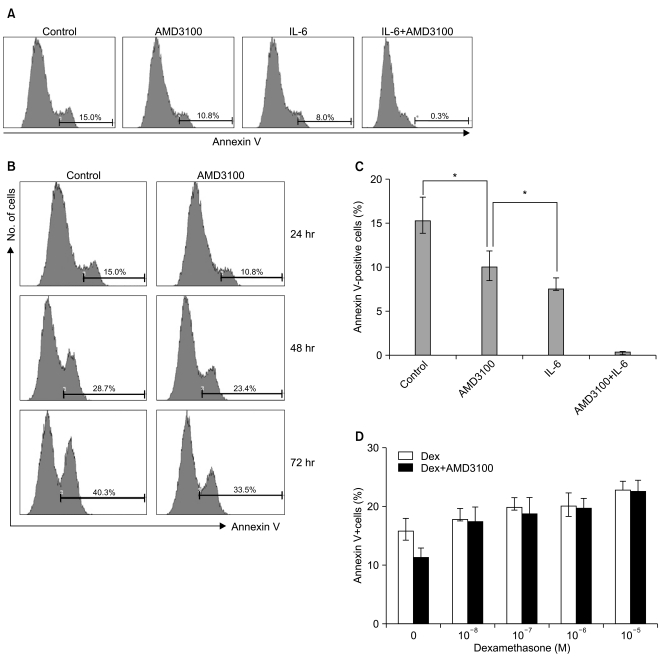
3. AMD3100 inhibits serum deprivation-mediated apoptosis of myeloma cells
To study whether AMD3100 has an effect on apoptosis in myeloma cells, RPMI8226 cells were incubated in RPMI-1640 medium without FBS. After 24 hours, 15.2±2.7% of the cells were annexin V-positive. AMD3100 slightly reduced this to 10.1±1.8%, and it further decreased to less than 1% with the addition of IL-6 (Fig. 3A). The apoptosis-reducing effects of AMD3100 were observed for up to 72 hours of incubation (Fig. 3B). Similar results were obtained with U266 cells (data not shown). Dexamethasone (10-7 to 10-5 M) minimally increased apoptosis in RPMI8226 cells; this increase was unaffected by AMD3100 (Fig. 3).
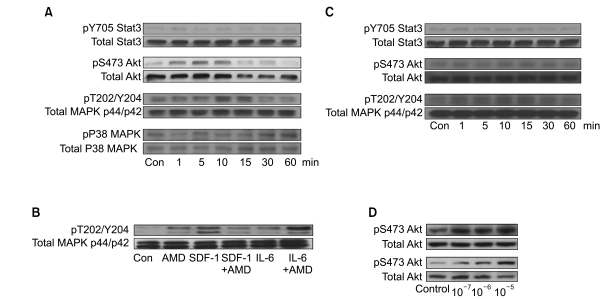
4. AMD3100 induces phosphorylation of signaling molecules in myeloma cells
We next examined whether AMD3100 induced the phosphorylation of Stat3, MAPK p38, Akt, and MAPK p44/p42, which are involved in SDF-1-mediated signaling (16), using RPMI8226 and U266 cells. Stat3, Akt and MAPK p44/p42 were all constitutively phosphorylated in these cell lines, to varying degrees. In U266 cells, 10-5 M AMD3100 alone enhanced the phosphorylation of Akt and MAPK p44/p42 to a modest degree, but not that of Stat3 or p38 MAPK. SDF-1 induced the phosphorylation of MAPK p44/p42, which was attenuated by AMD3100 (Fig. 4A). IL-6 enhanced the phosphorylation of MAPK p44/p42, which was further enhanced by AMD3100 (Fig. 4B). In RPMI8226 cells, AMD3100 alone slightly enhanced the phosphorylation of MAPK p44/p42, but not the phosphorylation of Stat3 or Akt (Fig. 4C). We then examined other cell types, including two hepatocellular carcinoma cell lines (PLC/PRF5 and Hep3B), to clarify whether the activation of the signaling molecules induced by AMD3100 was specific to myeloma cells. AMD3100 induced the phosphorylation of Akt in PLC/PRF5 and Hep3B (Fig. 4D), indicating that AMD3100-induced activation of these signaling molecules was not unique to myeloma cells.
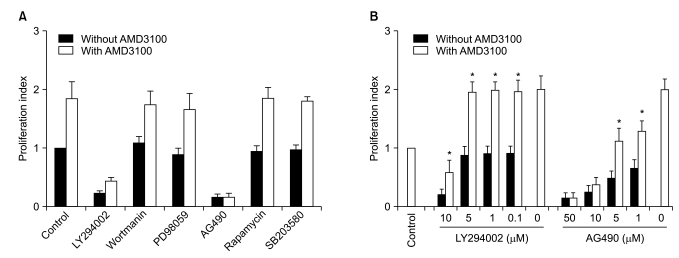
5. Influence of signal-blocking agents on AMD3100-induced proliferation of myeloma cells
To further define the signaling pathway(s) involved in the AMD3100-induced proliferation of myeloma cells, a proliferation assay was performed in the presence of various signal-blocking agents. First, RPMI8226 cells were incubated for 3 days with fixed concentrations of signal-blocking agents, which have been demonstrated to significantly inhibit signaling molecules and are thus commonly used in such experiments (10-5 M AMD3100, 10 µM LY294002, 100 nM wortmanin, 50 µM PD98059, 10 µM AG490, 50 nM rapamycin and 10 µM SB203580). The phosphatidyl inositol-3 (PI3) kinase inhibitor wortmanin, MAPK p44/p42 inhibitor PD98059, the mTOR inhibitor rapamycin, and the MAPK p38 inhibitor SB203580 did not affect AMD3100-induced or spontaneous proliferation in these cells. Another PI3 kinase inhibitor, LY294002, and a Jak2 inhibitor, AG490, completely inhibited not only AMD3100-induced proliferation, but also spontaneous proliferation of these cells (Fig. 5A). Thus, the signal-blocking effects of these agents were unclear.
To clarify the influences of these agents, we repeated the experiments using several lower concentrations of the agents. LY294002 at concentrations not inhibiting spontaneous proliferation (5 µM or less) also did not affect AMD3100-induced proliferation of the cells, whilst AG490 dose-dependently inhibited spontaneous proliferation and AMD3100-induced proliferation of the cells (Fig. 5B). Accordingly, the signal-blocking effects were still unclear. However, these results indicate that AMD3100 partially overcame the strong growth inhibition in myeloma cells induced by AG490.
6. AMD3100 induces a marked inhibition of survival and proliferation of myeloma cells during the late course in extended period cultures
Because a recent report demonstrated that AMD3100 induces a marked inhibition of survival in leukemic cells in culture for extended periods of up to 14 days (17), we further evaluated the effects of AMD3100 on the proliferation of myeloma cells in cultures of extended duration (up to 14 days). In controls, in which cells were incubated in serum-free medium alone, the number of cells increased for the first 5 days and then decreased. Again, AMD3100 at a high concentration (10-5 M) significantly enhanced proliferation of RPMI8226 cells over the first 5 days of incubation. However, after 5 to 7 days of incubation, AMD3100 induced a marked decrease in the number of cells compared to the control (Fig. 6A). Adding SDF-1 at the beginning and at day 7 of the incubation did not affect the early increase or subsequent decrease in the number of cells (Fig. 6B). To confirm the biphasic dual effects of AMD3100 on survival and growth of myeloma cells, we performed a tumor cell colony assay using RPMI8226 cells. Consistent with the proliferation assay, AMD3100 increased the number of colonies during the early period of the assay, but it markedly decreased the number and size of the colonies in later period of the assay (Fig. 6C and 6D). To define late effects of AMD3100 on apoptosis of myeloma cells, RPMI8226 cells were incubated in RPMI-1640 medium without FBS for up to 14 days. AMD3100 diminished cell apoptosis to a modest degree over the first 5 to 7 days, and then enhanced it compared with the controls (Fig. 7).
Discussion
AMD3100 is a well known specific antagonist of CXCR4, and does not interact with other chemokine receptors (18). Several studies have reported that AMD3100 blocks the binding of SDF-1 to CXCR4, resulting in inhibition of migration or proliferation induced by SDF-1 in many cell types (19,20). We report here for the first time that AMD3100 at a high concentration, for example 10-5 M (10 µM), stimulates the proliferation of myeloma cells in a short-term incubation for up to 5 days and protects cells from serum deprivation-induced apoptosis, which is puzzling given the well-known roles of the SDF-1/CXCR4 axis and AMD3100 in multiple myeloma (5-7,21). As myeloma cells produce and secrete SDF-1 (5), which may possibly acts as an autocrine growth factor, we initially expected AMD3100 to inhibit proliferation of myeloma cells. Unexpectedly, 10-5 M AMD3100 clearly stimulated the proliferation of myeloma cells in a short-term incubation for 3 days under serum-free conditions. The migration-blocking and CXCR4-internalizing effects indicate that AMD3100 is a specific antagonist of CXCR4. However, AMD3100 has also been demonstrated to activate a G protein coupled with CXCR4 and thus acts as a partial CXCR4 agonist in vitro (14,22). In the present study, we showed that AMD3100 alone induced the phosphorylation of some signaling molecules in myeloma cells. The proliferation-enhancing effects of AMD3100 in myeloma cells were abrogated by pretreating the cells with PTX, a strong G protein-coupled receptor antagonist. These results indicate that AMD3100 acts as a partial agonist for CXCR4 in myeloma cells. Phosphorylation of signaling molecules induced by AMD3100 was also observed in other cell types. AMD3100 induced the phosphorylation of Akt in two hepatocellular carcinoma cell lines, PLC/PRF5 and Hep3B, which express cell surface CXCR4 to a limited degree. Thus, the action of AMD3100 is not limited to myeloma cells.
We sought to define which signaling pathways were involved in the AMD3100-mediated proliferation of myeloma cells. Wortmanin and PD98059 did not affect the enhanced proliferation. In contrast, both LY294002 and AG490 completely blocked the proliferation of RPMI8226 cells at concentrations that are commonly used in signal-blocking experiments (23,24). Thus, the signal-blocking effects of these agents could not be explained; although AMD3100 partially overcame the inhibition of RPMI8226 cell proliferation induced by AG-490 dose-dependently.
A recent study showed that AMD3100 strongly impaired survival and stimulated the differentiation of leukemic cells, such as HL60 and U937, where these cells were incubated with AMD3100 at a concentration of 10-5 M for 7 to 9 days (17). Although the cells investigated in that study were different from those in our experiments, the results were apparently contradictory. Hence, we performed additional experiments in an attempt to clarify the apparent differences. RPMI8226 cells were incubated with AMD3100 under serum-free conditions for up to 14 days. We found that 10-5 M AMD3100 clearly enhanced proliferation of the cells during the early course of incubation. However, after 5 to 7 days of culture, the number of cells treated with AMD3100 decreased more rapidly as compared to control. A similar phenomenon was observed in U937 leukemic cells (data not shown). Thus, the results of the two studies did not actually differ. It seems that the investigators in the previous study overlooked the proliferation-enhancing effects of AMD3100 in leukemic cells in the early course of the incubation. Taken together, we suggest that AMD3100 alone induces signaling through CXCR4 and initially enhances proliferation and survival of myeloma cells, and then the SDF-1/CXCR4 blockade by AMD3100 induces subsequent rapid cell death later. Nevertheless the mechanism of AMD3100-induced cell death remains to be identified. Given that myeloma cells are a major source of plasma SDF-1 (5), a reasonable explanation could be that AMD3100 binding to CXCR4 results in a blockade of the autocrine loop of SDF-1. In the present study, we showed that PTX alone markedly inhibited the proliferation of myeloma cells. This result suggests that constitutive intrinsic signaling from G protein-coupled receptors, including CXCR4, may be important in the survival and proliferation of myeloma cells. Thus, additional explanation would be that AMD3100 binding to CXCR4 inhibited the intrinsic signaling derived from CXCR4.
Conclusion
AMD3100 has dual effects, initially enhancing and subsequently inhibiting, on survival and proliferation of myeloma cells via CXCR4 in vitro. Although the proliferation enhancement was modest and only apparent in the early period of incubation and when AMD3100 was added at a high concentration, we need to be cautious in the clinical application of this drug in patients with multiple myeloma. In contrast, the marked inhibition of survival in myeloma cells in the later period suggests a new therapeutic potential for AMD3100 in multiple myeloma. Further studies using in an in vivo experimental model are warranted.