AbstractPurposeGemcitabine plus cisplatin (GemCis) is the standard first-line chemotherapy for patients with advanced biliary tract cancer (BTC). In ABC-02 study, the BTC patients received up to 6-8 cycles of 3-weekly GemCis; however, those without progression often receive more than 6-8 cycles. The clinical benefit of maintenance treatment in patients without progression is uncertain.
Materials and MethodsAdvanced BTC patients treated with GemCis between April 2010 and February 2015 at Asan Medical Center, Seoul, Korea, were retrospectively analysed. The patients without progression after 6-8 cycles were stratified according to further treatment i.e., with or without further cycles of GemCis (maintenance vs. observation groups). The primary endpoint was overall survival (OS) and progression-free survival (PFS).
ResultsAmong the 740 BTC patients in the initial screen, 231 cases (31.2%) were eligible for analysis (111 in the observation group, 120 in the maintenance group). The median OS from the GemCis initiation was 20.5 months (95% confidence interval [CI], 15.4 to 25.6) and 22.4 months (95% CI, 17.0 to 27.8) in the observation and maintenance groups, respectively (p=0.162). The median PFS was 10.4 months (95% CI, 7.0 to 13.8) and 13.2 months (95% CI, 11.3 to 15.2), respectively (p=0.320).
IntroductionBiliary tract cancer (BTC) is a heterogeneous malignancy arising from the gallbladder, intrahepatic, and extrahepatic bile duct. Its incidence is quite rare in the United States with approximately 10,000 new diagnoses per year but is higher in Latin America and Asia [1,2]. Survival outcomes of BTC remain dismal with a 5-year overall survival (OS) rate of 30% for localized disease and 10% for patients with unresectable or metastatic disease [3,4].
In patients with unresectable or metastatic BTC, systemic chemotherapy is the standard of treatment. Gemcitabine plus cisplatin (GemCis) has been widely accepted as the appropriate first-line chemotherapeutic regimen for BTC based on the success of the previous pivotal phase III ABC-02 trial, which demonstrated the superiority of GemCis over gemcitabine monotherapy [5]. There is no standard second-line chemotherapy for BTC following a GemCis failure, although fluorouracil-based chemotherapy is commonly attempted [6].
In the ABC-02 trial, patients received first-line GemCis up to 24 weeks, which corresponded to a maximum of eight cycles, and then discontinued this treatment irrespective of their response. In real-world practice, however, patients who do not show progression during GemCis treatment are often continued on this chemotherapy beyond eight cycles until disease progression. However, the clinical benefit of maintenance therapy with GemCis is yet to be established.
In our present study, we retrospectively analysed the efficacy outcomes in BTC patients who were continued on a GemCis regimen after an initial disease control with 6-8 cycles of this treatment.
Materials and Methods1. PatientsPatients with histologically confirmed unresectable or metastatic BTC who received a first-line GemCis treatment at Asan Medical Center, Seoul, Korea, between April 2010 and June 2015, were identified and their medical records were retrospectively reviewed. Patients who had first-line chemotherapy other than GemCis or who were diagnosed with a combined histology of hepatocellular carcinoma and cholangiocarcinoma or with ampulla of Vater cancer were excluded from further analysis. Patients who showed a complete response (CR), partial response (PR), or stable disease (SD), as defined by Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1, after completion of a 6-8 cycle first-line GemCis therapy were included in the study population. These cases were classified into a maintenance group (continued on GemCis beyond eight cycles) and observation group (GemCis was discontinued after a 6-8 cycle first-line regimen).
2. TreatmentAll of the study patients had received the GemCis regimen described previously i.e. cisplatin at 25 mg/m2 followed by gemcitabine at 1,000 mg/m2 on days 1 and 8, every 3 weeks. Responses to treatment were evaluated every two or three cycles using computed tomography or magnetic resonance imaging and graded according to RECIST ver. 1.1. In patients who showed disease control (CR, PR, or SD) after completion of 6-8 cycles of GemCis, continuation on GemCis as a treatment maintenance was considered at the discretion of the attending physicians and with shared decision-making under conditions of uncertainty. In the observation group, patients were not continued on GemCis and had a regular imaging follow-up every 6-8 weeks. Patients in the maintenance group were continued on GemCis until disease progression or unacceptable adverse events due to toxicity. Best supportive care was provided for all patients.
Subsequent chemotherapy in patients showing disease progression was determined by the treating physicians. In the observation group, resumption of GemCis was allowed for patients who showed a > 6 month progression-free interval between the last chemotherapy dose and disease progression.
3. StatisticsComparison of survival outcomes between two treatment groups was performed in terms of OS and progression-free survival (PFS). OS from the initiation of treatment was defined as the period from the commencement of the firstline GemCis to death from any cause. PFS from the initiation of treatment was defined as the time from the first-line Gem-Cis to disease progression or death, whichever occurred first. OS from the completion of scheduled GemCis was defined as the time from the end of last cycle of GemCis in the observation group, and the cycle 9 day 1 of GemCis in the maintenance group to any cause of death. PFS from the completion of scheduled GemCis was defined as the period from the end of last cycle of GemCis in the observation group, and the cycle 9 day 1 of GemCis in the maintenance group to disease progression or any cause of death. OS and PFS curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate analyses for the OS and PFS outcomes were performed using a Cox proportional hazards model. A two-sided p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS ver. 21.0 (IBM Corp., Armonk, NY).
4. Ethical statementAll procedures involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration. This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (2015-0684). IRB waived informed consent for this study because of its nature of retrospective analysis.
Results1. Baseline characteristics of the study patientsA total of 740 patients with advanced BTC who previously received GemCis as first-line chemotherapy were identified on our institutional database. Among these cases, 231 (31.2%) patients did not progress, as defined by RECIST ver. 1.1, after completion of 6-8 cycles of GemCis. This included 111 patients (48.1%) who did not receive further GemCis (observation group) and 120 cases (51.9%) who received further Gem-Cis as a maintenance therapy (maintenance group) (Fig. 1). The baseline characteristics of these patients are summarized in Table 1.
There were no significant differences in baseline characteristics between these two groups except for the Eastern Cooperative Oncology Group performance status, number of metastatic sites, and best response to first-line GemCis. Patients in the maintenance group showed higher objective response rates compared to those in the observation group (27.5% vs. 14.4%, p=0.015).
2. First-line GemCisA median of six and 14 cycles of GemCis was administered in the observation and maintenance groups, respectively. In the maintenance group, median six cycles (range, 1 to 34) of additional GemCis were administered after completion of scheduled eight cycles. Among these patients, 51 patients (42.5%) had gemcitabine monotherapy from cycle 9, and 69 patients (57.5%) received at least one dose of cisplatin (median, 2 cycles; range, 1 to 17) during maintenance GemCis.
In the observation group, 76 patients (68.5%) were discontinued on GemCis due to the completion of the planned chemotherapy, 27 patients (24.3%) due to the patient’s request or adverse events from toxicity, and eight patients (7.2%) were lost to follow up. In the maintenance group, 66 patients (55%) discontinued GemCis due to disease progression, 13 patients (10.8%) due to patient’s request or adverse events from toxicity, eight patients (6.7%) with physician’s decision, and seven patients (5.8%) were lost to follow-up. Twenty-six patients (21.7%) had on-going GemCis at the time of data collection.
3. Survival outcomes from the initiation of first-line GemCisIn the overall study patient population, the median followup period was 23.8 months (interquartile range, 5.1 to 86.3 months), the median OS from the initiation of treatment was 22.3 months (95% confidence interval [CI], 19.0 to 25.7) and the median PFS from the initiation of treatment was 12.5 months (95% CI, 11.1 to 13.9). The median PFS and OS of the observation group were 10.4 months (95% CI, 7.0 to 13.8) and 20.5 months (95% CI, 15.4 to 25.6), respectively. In the maintenance group, the median PFS and OS were 13.2 months (95% CI, 11.3 to 15.2) and 22.4 months (95% CI, 17.0 to 27.8), respectively. There were no statistically significant differences in PFS (p=0.320) and OS (p=0.162) between the two groups (Fig. 2). These findings were consistent with the results of multivariate analyses including other potential prognostic factors (Table 2).
4. Survival outcomes from the completion of scheduled GemCisIn the overall study patient population, the median OS and PFS from the completion of scheduled GemCis was 16.9 months (95% CI, 12.7 to 21.1), and 6.8 months (95% CI, 5.9 to 7.7). There was no statistically significant difference of survival outcomes between two groups in terms of OS with median OS of 14.4 months (95% CI, 7.6 to 21.3) and 16.9 months (95% CI, 11.5 to 22.3) in the observation group, and the maintenance group, respectively (p=0.290) (Fig. 3A). The median PFS was significantly longer in the maintenance group with 7.8 months (95% CI, 6.6 to 9.0) compared to 5.2 months (95% CI, 3.5 to 7.0) of the observation group (p=0.016) (Fig. 3B).
5. Second-line treatmentAfter disease progression on first-line GemCis, 45 (43.7%) and 50 (53.8%) patients subsequently received a second-line chemotherapy in the observation and maintenance groups, respectively. Most of these patients (n=78, 82.1%) received fluoropyrimidine-based regimens as second-line chemotherapy. GemCis was re-administered in 15 of the 45 patients (33.3%) with progression in the observation group (Fig. 1). The median PFS with second-line chemotherapy was longer in the observation group than in the maintenance group (4.9 months [95% CI, 2.6 to 7.2] vs. 2.6 months [95% CI, 2.1 to 4.8]; p=0.012) (Fig. 4). However, the median OS in the second-line setting did not differ between these groups (12.0 months [95% CI, 15.4 to 25.6] and 8.3 months [95% CI, 5.6 to 10.9]; p=0.135) (Fig. 4).
In further subgroup analyses according to second-line chemotherapy regimens (S2 Fig.), the survival outcomes were significantly longer in patients who had readministration of GemCis in the observation group (Re-GemCis group) compared to the patients who received second-line fluoropyrimidine-based chemotherapy in the observation and maintenance groups with median OS of 45.3 months, 9.9 months, and 8.3 months, respectively (Re-GemCis observation group vs. fluoropyrimidine observation group, p=0.028; Re-GemCis observation group vs. maintenance group, p=0.020). PFS was also significantly longer in Re-GemCis group with median PFS of 7.0 months, 3.1 months, and 2.7 months, respectively (Re-GemCis observation group vs. fluoropyrimidine observation group, p=0.001; Re-Gemcis observation group vs. maintenance group, p < 0.001). Survival outcomes of patients with second-line fluoropyrimidine-based therapy were similar between the maintenance and observation groups (p=0.680 for OS and p=0.780 for PFS).
DiscussionIn this retrospective analysis of 231 BTC patients who did not progress after the completion of a 6-8 cycle first-line GemCis, our findings suggest that there is no significant clinical benefit from maintenance therapy in BTC patients for whom durable disease control has been achieved. Our results also showed that the patients with durable response to firstline GemCis until 6-8 cycles may have prolonged survival as median OS was 22.3 months from the initiation of GemCis in our cohort.
In our current study, there were no significant differences in PFS or OS between the observation and maintenance groups (p=0.320 and p=0.162, respectively), although the maintenance group showed a slightly improved median PFS (13.2 months vs. 10.4 months) and OS (22.4 months vs. 20.5 months). This finding was consistent even after adjustment for potential confounding factors in the multivariate analysis.
Our findings are on the contrary to the results of a recent retrospective study which included 396 patients with advanced gallbladder cancer [7]. In this study, 70 patients among 120 patients who did not progress after 6-8 cycles of first-line treatment had maintenance therapy and OS was significantly longer in patients with maintenance chemotherapy compared to those without (median, 14.88 months vs. 10.87 months; p=0.033). However, this study is limited in terms of the inclusion of only patients with gallbladder cancer and heterogeneous first-line chemotherapy regimens as 56.6% of patients received gemcitabine plus oxaliplatin. In addition, the survival difference shown in this study was not tested in the multivariate analysis, which may not exclude the potential impact of confounding factors.
Continuation of long-term cytotoxic chemotherapy for more than 6 months results in cumulative toxicities that may ultimately impair the quality of life of the treated patient. Thus, for patients in whom durable disease control (at least SD) is achieved with chemotherapy, a chemotherapy-free period with careful follow-up may be a reasonable strategy in the management of unresectable or metastatic BTC. This is considering the lack of a significant survival benefit from maintenance chemotherapy approach that we observed from our current analysis. This is also supported by the fact that there was no significant difference in OS from the completion of scheduled GemCis between two groups, despite the longer PFS from the completion of scheduled GemCis seen in the maintenance group.
The clinical benefits of continuing palliative chemotherapy in patients with a sustained response have been investigated previously in multiple cancer types. In colorectal cancer, there is clinical evidence which favours a drug holiday period from cytotoxic chemotherapy in patients with a sustained response [8-10]. In addition, a report on patients with metastatic colorectal cancer has indicated that intermittent FOLFIRI approach (a 2-month drug holiday every four cycles) may provide comparable survival outcomes to a continuous FOLFIRI regimen (18.0 months vs. 17.0 months; hazard ratio, 0.88) [11]. In patients with non small-cell lung cancer, four cycles of platinum-doublet chemotherapy was no-inferior to six cycles of chemotherapy [12]; however, the maintenance therapy with pemetrexed following induction pemetrexed plus cisplatin demonstrated improved survival compared to placebo [13]. In patients with metastatic breast cancer, maintenance treatment with gemcitabine plus paclitaxel after induction six cycles of chemotherapy showed better PFS and OS compared with observation [14]. These suggest that the clinical relevance of maintenance chemotherapy may differ according to the tumor type and chemotherapy agents.
In patients who received second-line treatment, PFS with second-line treatment was significantly longer in the observation group, while there was no statistical difference in OS between two groups. This might be interpreted as the potential negative impact of maintenance therapy to second-line treatment due to the cumulative toxicity and deterioration of patient performance with long-standing chemotherapy. However, there was no significant difference in the proportion of receiving second-line chemotherapy after progression on GemCis between two groups and the difference in PFS with second-line therapy between two groups may be derived from the patients who had re-administration of GemCis in the observation group. With exclusion of patients with re-administration of GemCis, there was no significant difference in PFS and OS with second-line treatment between two groups. Because GemCis was re-administered only in patients who had at least 6 months of progression-free interval in the observational group, better survival with these patients may reflect their indolent disease courses. This is in line with the results of previous prospective study for metastatic breast cancer, as duration of first-line chemotherapy did not have impact on the treatment exposure on subsequent second-line chemotherapy [14].
Our current study has inherent limitations due to the retrospective nature of the analyses and single centre population. Our analysis also had strengths, however, as the study population was a homogeneous series of patients who all received a standard GemCis first-line treatment and was of sufficient size to conduct comparative analysis for subgroup and multivariate analysis.
In conclusion, the maintenance of GemCis for advanced BTC patients in whom disease control has been achieved after six to eight cycles may not produce a survival benefit. Considering the cumulative toxicities and impact on quality of life from the sustained administration of chemotherapeutic drugs, a stop-and-go strategy may be more appropriate in this patient population. Further validation of this possibility is needed in a future prospective randomized trial.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
AcknowledgmentsThis study was supported in part by the Bio and Medical Technology Development Program of the NRF funded by the Korean government, MSIP (NRF-2016M3A9E8941331).
Fig. 1.Study outline. BTC, biliary tract cancer; GemCis, gemcitabine plus cisplatin; PD, progressive disease. 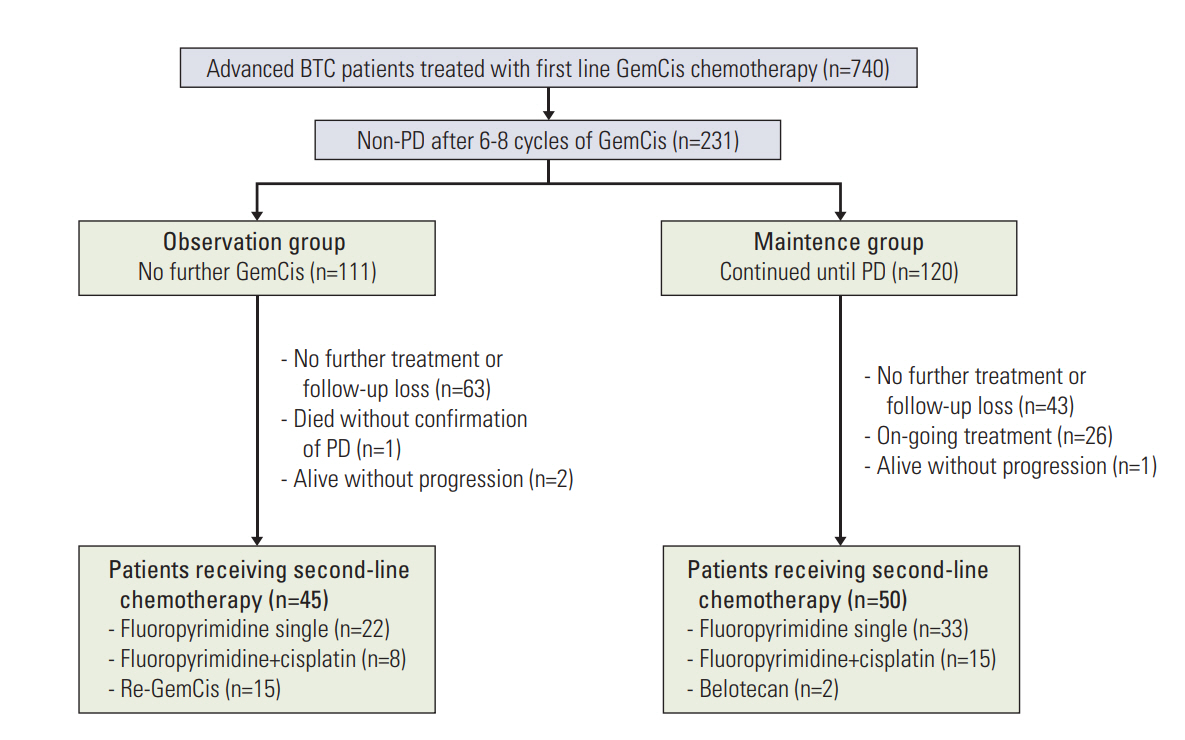
Fig. 2.Overall survival (OS) (A) and progression-free survival (PFS) (B) from the initiation of first-line gemcitabine plus cisplatin. CI, confidence interval. 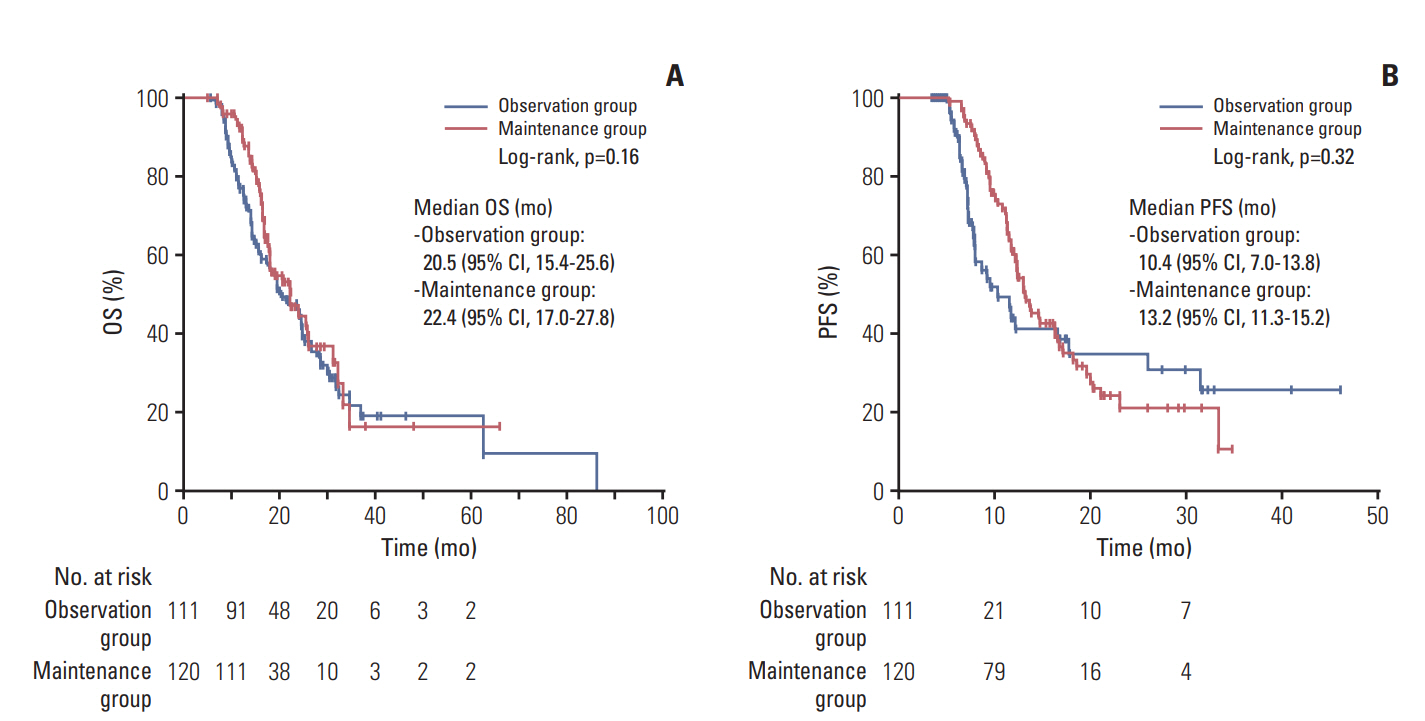
Fig. 3.Overall survival (OS) (A) and progression-free survival (PFS) (B) from the completion of scheduled gemcitabine plus cisplatin. 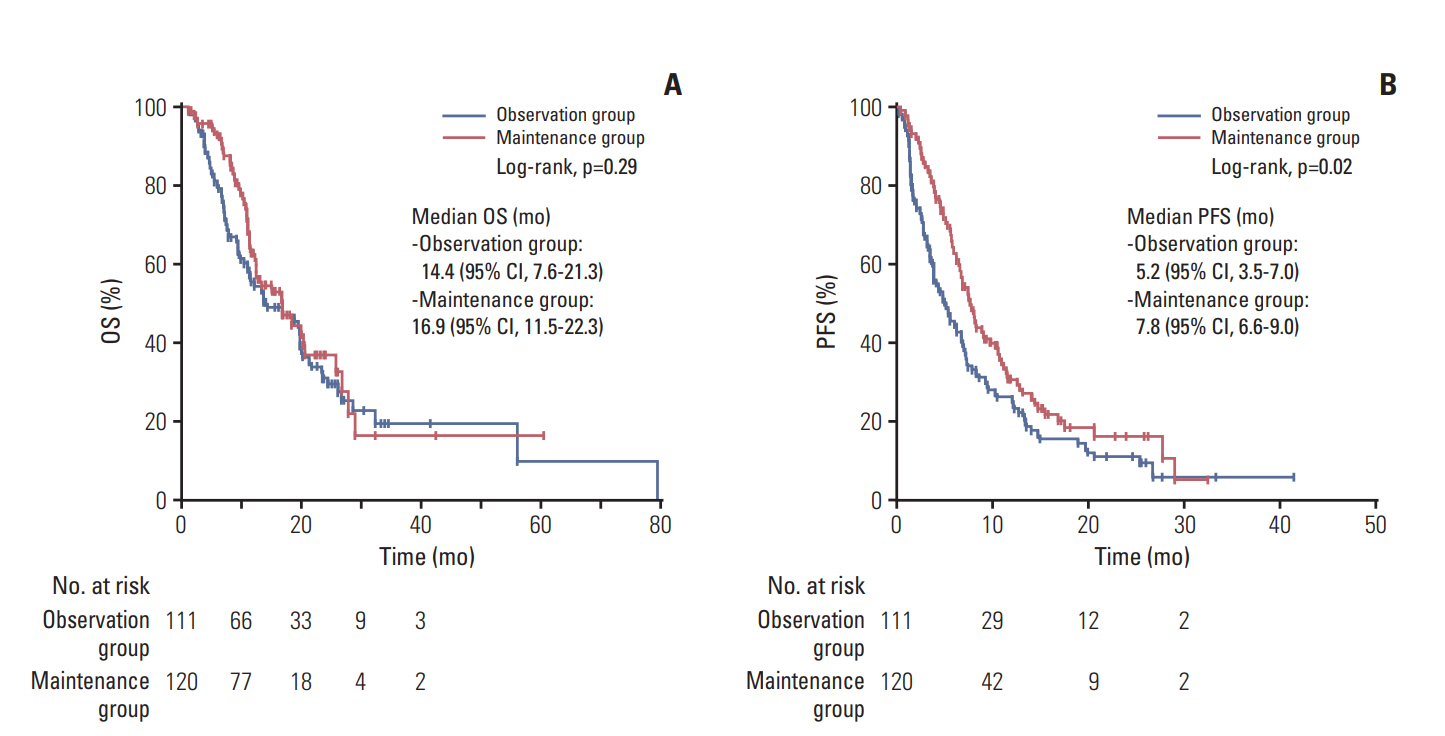
Fig. 4.Overall survival (OS) (A) and progression-free survival (PFS) (B) with second-line therapy after disease progression on first-line gemcitabine plus cisplatin. 
Table 1.Baseline clinical characteristics of the study patients
Table 2.Univariate and multivariate analysis of prognostic factors for overall survival from the initiation of first-line Gem-Cis HR, hazard ratio; CI, confidence interval; IHCCA, intrahepatic cholangiocarcinoma; EHCCA, extrahepatic cholangiocarcinoma; ECOG, Eastern Cooperative Oncology Group; GemCis, gemcitabine plus cisplatin; CR, complete response; PR, partial response; SD, stable disease; CA 19-9, carbohydrate antigen 19-9. References1. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–7.
3. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER cancer statistics review, 1975-2013. Bethesda, MD: National Cancer Institute; 2016.
4. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
5. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81.
6. Kim BJ, Yoo C, Kim KP, Hyung J, Park SJ, Ryoo BY, et al. Efficacy of fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: retrospective analysis of 321 patients. Br J Cancer. 2017;116:561–7.
7. Ostwal V, Pinninti R, Ramaswamy A, Shetty N, Goel M, Patkar S, et al. Treatment of advanced Gall bladder cancer in the real world-can continuation chemotherapy improve outcomes? J Gastrointest Oncol. 2017;8:368–76.
8. Maughan TS, James RD, Kerr DJ, Ledermann JA, Seymour MT, Topham C, et al. Comparison of intermittent and continuous palliative chemotherapy for advanced colorectal cancer: a multicentre randomised trial. Lancet. 2003;361:457–64.
9. Tonini G, Imperatori M, Vincenzi B, Frezza AM, Santini D. Rechallenge therapy and treatment holiday: different strategies in management of metastatic colorectal cancer. J Exp Clin Cancer Res. 2013;32:92.
10. Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol. 2006;24:394–400.
11. Labianca R, Sobrero A, Isa L, Cortesi E, Barni S, Nicolella D, et al. Intermittent versus continuous chemotherapy in advanced colorectal cancer: a randomised 'GISCAD' trial. Ann Oncol. 2011;22:1236–42.
12. Park JO, Kim SW, Ahn JS, Suh C, Lee JS, Jang JS, et al. Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25:5233–9.
13. Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–902.
14. Park YH, Jung KH, Im SA, Sohn JH, Ro J, Ahn JH, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol. 2013;31:1732–9.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||