Treatment of Non-small Cell Lung Carcinoma after Failure of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor
Article information
Abstract
Since the first description of non-small cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutation as a distinct clinical entity, studies have proved EGFR tyrosine kinase inhibitors (TKIs) as a first choice of treatment. The median response duration of TKIs as a first-line treatment for EGFR mutant tumors ranges from 11 to 14 months. However, acquired resistance to EGFR-TKIs is inevitable due to various mechanisms, such as T790M, c-Met amplification, activation of alternative pathways (IGF-1, HGF, PI3CA, AXL), transformation to mesenchymal cell or small cell features, and tumor heterogeneity. Until development of a successful treatment strategy to overcome such acquired resistance, few options are currently available. Here we provide a summary of the therapeutic options after failure of first line EGFR-TKI treatment for NSCLC.
Introduction
Since the first description of non-small cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutation as a distinct clinical entity [1], studies have proved EGFR-tyrosine kinase inhibitors (TKIs) as a first choice of treatment. [2-4]. The median response duration of TKIs as first line treatment for EGFR mutant tumors ranges from 11 to 14 months [2-5].
However, acquired resistance to EGFR-TKIs is inevitable due to various mechanisms, such as T790M, c-Met amplification, activation of alternative pathways (IGF-1, HGF, PI3CA, AXL [6]), transformation to mesenchymal cells [7] or small cell features [8], and tumor heterogeneity [9]. A randomized trial comparing the irreversible ErbB-family blocker afatinib versus placebo in patients with NSCLC after prior EGFR-TKI exposure failed to prove a benefit of overall survival [10]. Another trial comparing dacomitinib versus placebo in a similar clinical setting is ongoing (NCT01000025). Until development of a successful treatment to overcome such acquired resistance, few options are currently available.
Here we provide a summary of the therapeutic options after failure of first line EGFR-TKI treatment for NSCLC.
Change to Other Chemotherapeutic Agents
Because the performance status of patients after failure of first line EGFR-TKI tends to be relatively good, most patients are expected to receive second line treatment. After failure of first line EGFR-TKI, docetaxel, pemetrexed, or platinum doublet with or without bevacizumab can be used as a second line treatment [11]. Pemetrexed is favored because of its low toxicity and relatively good efficacy in EGFR mutant tumors [12]. In cases of squamous cell carcinoma harboring EGFR mutation, pemetrexed and bavacizumab are not indicated.
A chemotherapy regimen capable of achieving long-term disease control after failure of EGFR-TKI would be very helpful. However, no prospective trial comparing the efficacy of these regimens in such a clinical setting has been conducted. A retrospective analysis comparing the efficacy of taxane platinum doublet versus gemcitabine platinum doublet as first line treatments for NSCLC with or without EGFR mutation has been reported [13]. In patients with wild type EGFR, no significant difference was observed in response rate and progression free survival. However, in patients with EGFR mutation, progression free survival of taxane doublet was significantly superior to that of gemcitabine doublet. However, to the best of the author's knowledge, no study for prospective comparison of second line cytotoxic chemotherapy regimens after failure of first line EGFR-TKI has been reported.
A prospective phase II study comparing AUY922 (heat shock protein 90 inhibitor, Novartis) versus docetaxel or pemetrexed in patients who had responded to prior EGFR-TKI and harboring the EGFR mutation (ClinicalTrials.gov, no. NCT01646125) is ongoing.
The importance of re-biopsy of relapsed tumor tissue has recently been emphasized [8]. Because development of acquired resistance to EGFR-TKIs can occur through various mechanisms, such as T790M, c-Met amplification, activation of alternative pathways (IGF-1, HGF, PI3CA, AXL [6]), transformation to mesenchymal cell or small cell features [8], and tumor heterogeneity, molecular studies on relapsed tumor tissues is the most important step in combatting resistance.
Continuation of EGFR-TKIs with or without Other Therapy
1. Asymptomatic progressive disease
When the disease shows progressive disease according to response evaluation criteria such as Response Evaluation Criteria In Solid Tumors (RECIST) [14], the common practice is to switch to a different treatment regimen. However, the National Comprehensive Cancer Network (NCCN) treatment guideline for NSCLC [11] amended the recommendations for continuous use of EGFR-TKI even after development of acquired resistance.
This strategy is supported by the following lines of evidence. When MET amplification is the cause of progression, a MET inhibitor is added to the EGFR inhibitor (MetLung trial, no. NCT01456325). Thus, in order to overcome resistance, EGFR must still be inhibited. In addition, discontinuation of EGFR-TKI can lead to development of "disease flare," a much more accelerated progression of the tumor [15, 16].
Yang et al. [17] recently grouped the pattern of progression after EGFR-TKI as a dramatic, gradual, and local progression. Continuation of EGFR-TKI provided better results than switching to cytotoxic chemotherapy for the gradual progression group. However, they also suggested chemotherapy for the dramatic group with rapid tumor increment, and continuation of EGFR-TKI and local treatment for the local progression group.
2. Progression of brain metastasis
Sometimes, despite adequate control of extracranial tumors, lung cancer shows progression from the brain or leptomeningeal lesions. Jackman et al. [18] reported on a patient who showed isolated progression of leptomengeal metastasis while taking gefitinib 250 mg/day and showed subsequent regression with a higher dose of gefitinib 1,000 mg/day. The cerebrospinal fluid (CSF) concentration of gefitinib 250 mg/day was less than the level required for inhibition of growth of a lung cancer cell line in vitro. When the gefitinib dose was increased to 1,000 mg/day, the resulting CSF concentrations of gefitinib increased above the inhibitory concentration and eventually showed correlation with clinical response.
In the case of crizotinib, which is being used for lung cancer harboring EML4-ALK gene rearrangement, the CSF concentration of crizotinib was very low compared to its plasma concentration [19]. The low drug level in the CSF implies poor blood-brain barrier penetration of TKIs. Thus, the NCCN guideline also recommends continuing EGFR-TKI and addition of local therapy or whole brain radiation treatment [11].
3. Symptomatic relapse of an isolated extra-cranial lesion
Localized relapse such as single supraclavicular lymph node or isolated bony metastasis may be controlled with radiation treatment. In such cases, local treatment with continuing EGFR-TKI can be considered [11]. In a recent report [20] on 18 patients with extracranial local progression, local therapy with continued treatment with an EGFR-TKI was shown to be well tolerated and showed an association with long progression free survival (PFS) and overall survival.
Switch or Retreatment of EGFR-TKIs Following a Drug Holiday
Most patients who show response to EGFR-TKIs eventually experience disease progression after a median of 12 months. Instead of changing to another chemotherapeutic regimen, the same or a different EGFR-TKI can be re-introduced. In a prospective trial of 10 patients who had previously responded to erlotinib or gefitinib, Riely et al. [16] reported that these patients continued to benefit from retreatment with EGFR-TKI despite documented progression of the disease by RECIST. When patients with acquired resistance to EGFR-TKI discontinued EGFR-TKI treatment, the majority of patients experienced worsening lung cancer symptoms, increased tumor size, and increased fludeoxyglucose (FDG) uptake. Just three weeks after resumption of EGFR-TKI, the majority of patients showed stabilization or improvement of symptoms, decreased tumor size, and reduced tumor FDG uptake.
In several retreatment trials, a switch was made from gefitinib to erlotinib, and in some trials, the same EGFR-TKI was used after a drug holiday or treatment with another line of cytotoxic chemotherapy.
1. Switching from gefitinib to erlotinib
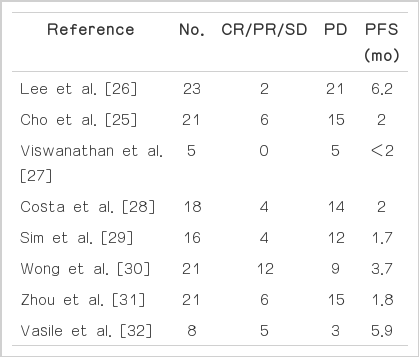
The recommended daily dose of elotinib is near its maximum tolerated dose (MTD) [21]; however, that of gefitinib is only one third of its MTD [22]. Thus, toxicities are more frequent and higher with erlotinib compared with gefitinib. In addition, erlotinib showed survival benefits in squamous cell carcinoma or smokers [23], while gefitinib failed to do so [24]. Thus, in several studies, erlotinib was tried after disease progression due to acquired resistance to gefitinib. Cho et al. [25] reported a response rate of 9.5% and a disease control rate (DCR) of 28.6% among 17 patients who had been previously treated with gefitinib and showed subsequent progression. Other trials have reported a DCR of up to 63% and a median progression free survival of 1.7-6.2 months (Table 1) [25-32].
2. Retrial of the same EGFR-TKI after a drug holiday
Possible differences in the potency of EGFR-TKIs cannot explain every case of second response after re-administration of EGFR-TKI. Similar responses were observed when the same EGFR-TKI was re-used after a drug holiday or treatment with another line of chemotherapy.
Becker et al. [33] reported a response rate of 36% and a DCR of 86% with a second round of erlotinib after a drug holiday (median, 9.5 months). Several trials have reported results of gefitinib retrial after a drug holiday or treatment with another line of chemotherapy. Yokouchi et al. [34], who conducted a retrospective analysis of nine patients, reported a DCR of 89%. Oh et al. [35] prospectively enrolled 20 patients whose tumor had progressed after failure of gefitinib treatment followed by treatment with another line of chemotherapy. After re-introduction of gefitinib, 25% achieved partial remission and 75% showed DCR. However, Asahina et al. [36] reported a DCR of only 44% and a PFS of 2.5 months (Table 2) [33-36].
A different response to the same EGFR-TKI regimen after a drug holiday can be explained with tumor heterogeneity. Repopulation of sensitive clones to EGFR-TKI may show a different response to EGFR-TKI in retrial (Fig. 1). In a retrospective analysis of an erlotinib retrial, Becker et al. [33] reported five cases of the T790M mutation and exon 19 deletions in rebiopsy specimens taken before the retrial of erlotinib. Among the five patients with the T790M mutation, two showed partial remission, and one showed stable disease. A selection process due to chemotherapy or to lack of EGFR-TKI may lead to a reduction in the fraction of tumor cells with additional T790M compared to cells exhibiting the activating mutation.

Schematic tumor volume-time curve according to on-and-off of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI). Tumor volume is the sum of EGFR-TKI sensitive clones and resistant clones regardless of their molecular mechanisms. The number of EGFR-TKI sensitive clones shows a rapid decrease after initiation of EGFR-TKI, however, that of EGFR-TKI resistant clones shows a steady increase despite EGFR-TKI treatment. Initially, tumor volume reaches partial response (PR) due to decrease of EGFR-TKI sensitive clones, but eventually shows progressive disease (PD) with the increase of EGFR-TKI resistant clones. If EGFR-TKI is discontinued at disease progression, explosive growth of EGFR-TKI sensitive clones can cause disease flare. If EGFR-TKI is reintroduced, EGFR-TKI sensitive clones show a decrease again, and tumor volume can be stabilized (decreasing stable disease [SD] or PR). However, because of increasing EGFR-TKI resistant clones, the duration of tumor volume stabilization would be shorter than that of initial treatment with EGFR-TKI.
Thus, retreatment with the same EGFR-TKI regimen is an option for patients with NSCLC who initially responded to EGFR-TKI treatment and recurred after a drug holiday or treatment with another line of cytotoxic chemotherapy. In view of tumor heterogeneity, resistance mutations such as T790M do not exclude response to EGFR-TKI retreatment.
Next generation of EGFR-TKIs and Combination Strategies
Several strategies for overcoming resistance have been suggested. The ability of irreversible pan-HER inhibitors such as afatinib and dacomitinib to overcome T790M-mediated resistance to gefitinib has been demonstrated in some preclinical studies; therefore, they are being actively investigated.
Dacomitinib showed a better response rate and prolonged response duration compared to erlotinib in patients without prior EGFR-targeted therapy [37]. Similarly, compared with previous studies with first line gefitinib or erlotinib, numerically longer median PFS was achieved by afatinib in EGFR-TKI-naïve patients [38]. However, these second-generation EGFR-TKIs would have limitations in overcoming resistance because results of clinical trials in patients with acquired resistance to EGFR-TKIs have been disappointing [39].
Novel pyrimidine-based, mutant-selective EGFR inhibitors were recently discovered by pharmacological screening of a library of compounds [40]. These are more potent against mutant EGFR regardless of T790M, and less potent against wild type EGFR compared to quinazoline-based EGFR-TKIs, suggesting that use of these drugs could be a promising strategy for treatment of EGFR-TKI resistance.
Another approach for T790M-mediated resistance is the dual inhibition of EGFR with afatinib and cetuximab, an EGFR blocking antibody resembling the synergistic combination of lapatinib and trastuzumab in ErbB2-positive breast cancer. Due to promising preliminary results of activity and tolerability, final results of this regimen are being eagerly anticipated [41]. In selected patients with acquisition of resistance by activated compensatory pathways such as MET and AXL, addition of a MET [42] or AXL [6] inhibitor to EGFR-TKIs could be beneficial.
Conclusion
Development of disease progression eventually occurs after a median duration of 11-14 months after EGFR-TKI treatment in NSCLC harboring activating EGFR mutations. If the progression is localized, continued use of EGFR-TKI along with local treatment such as radiation is recommended. If the tumor shows systemic progression, switching to cytotoxic chemotherapy with or without EGFR-TKI is an option. After a drug holiday or cytotoxic chemotherapy, a second round of EGFR-TKI treatment may show another response.
Notes
Conflict of interest relevant to this article was not reported.