Molecular Epidemiology of Colon Cancer
Article information
Abstract
Colorectal cancer appears to have rapidly increased over the past two decades in Korea. Environmental factors, characterized by a western life style, seem to be closely related to the increased risk of colorectal cancer. Higher intakes of meat, a lower vegetable intake, a lack of physical activity, obesity, and alcohol drinking have been suggested to be risk factors for colorectal cancer in the numerous epidemiologic studies. Several specific associations have also been observed between genetic polymorphisms and colorectal cancer. Moreover, it has been postulated that environmental factors and a genetic predisposition work in concert in colorectal cancer development. A stronger association between red meat intake and colorectal cancer among those with rapid acetylators at either the NAT1 or NAT2 locus was reported, particularly for colorectal cancer associated with K-ras mutations. The protective effect of the homozygous variant TT form of the MTHFR genotype on the risk of colon cancer seems to be modified by the level of methyl diets, i.e., by folate, which has a protective effect, or conversely by alcohol. The insulin-related pathway, which possibly explains at a mechanistic level the effect of physical activity and obesity on colon cancer, appears to be a common denominator in colon cancer and in other metabolic disorders, such as diabetes mellitus and dyslipidemia. Hyperinsulinemia has been proposed as an explanation for the association between a Western lifestyle and colon cancer risk. Further studies, that incorporate both genetic and environmental factors, are needed to fully explain and identify the underlying pathway of colorectal carcinogenesis.
INTRODUCTION
Colorectal cancer is the fourth most common cancer and accounts for 8.5% of all incident cases worldwide (1). Age-standardized incidence rates, however, vary by 20-fold across countries. The highest rates occur in Western societies, such as, in North America, Western Europe, Australia, and New Zealand (30~50 per 100,000), while rates were low in most Asian and African countries (less than 10 per 100,000) in the late 1980s. However, recently incidences have increased in some Asian countries like Japan (2). According to the recent incidence statisties (3), the highest incidence was reported in Hiroshima, Japan (86.7 per 100,000 in men). The incidence of colorectal cancer have also increased rapidly over the past two decades in Korea, which was previously known as low risk area. Age-standardized mortality rates in Korea increased from 2.4 in 100,000 to 10.2 in 100,000 for men and from 1.8 in 100,000 to 6.0 in 100,000 for women between 1983 and 2000 (4). Moreover, the increased mortality due to colorectal cancer over the same period appears to be steeper than that of lung cancer in men and women equally (Fig. 1). Based on the Centra Cancer Registry Program (CCRP), a hospital-based cancer registry operated by the Korean government since 1980, the proportion of colorectal cancer case among total registered cancers increased in men and women from 5.8% and 5.8% in 1983 to 11.6% and 10.7% in 2002, respectively (5).
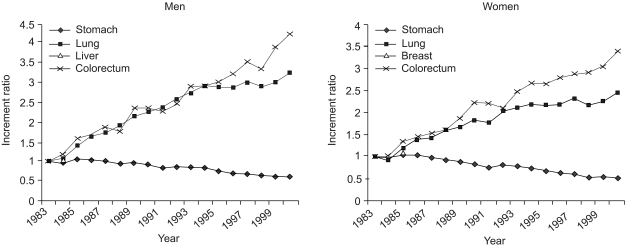
Increment ratios of mortality rates for selected cancers in Korea for succeeding years versus 1983 rates by sex.
Studies on migrants populations showed that those moving from low- to high- risk countries acquired an elevated risk of large bowel cancer even in the first-generation (6,7). It was proposed that environmental factors, characterized by a western life style, are closely related to the risk of colorectal cancer (8). Numerous studies have suggested that a higher intake of red meat, and possibly in association with the cooking process, may increase risk of colorectal cancer. In addition, it has been consistently reported that physical activity and obesity are associated with colorectal cancer risk. Recently, methyl diets, such as, folate and alcohol intake, have also be related to colon cancer risk. And, several studies have noted a higher risk of colon cancer among smokers, especially among those with long smoking histories.
There appears to be a familial aggregation in colorectal cancer, which suggests that a genetic predisposition may be important in the etiology of the disease. A number of associations have also been observed between specific genetic polymorphisms and colorectal cancer. It was argued that most colorectal cancers are neither purely genetic nor purely environmental (9). Environmental factors, both dietary and other environmental factors, appear to interact with genetic factors in the development of colorectal cancer.
In this review, we present how some established environmental factors interact with genetic factors to modify the risk of colorectal cancer.
Environmental Factors and Related Genetic Factors
1) A Higher Intake of Red Meat and Related Genetic Susceptibility
Rate of colon cancer was reported to be strongly correlated with the national per capita consumption of animal fat in one large scale international ecologic study (10). The majority of epidemiologic studies have shown a positive association with intake of red meat (8), with some exceptions (11). The effect estimates between red meat and colon cancer for some selected populations are shown in Table 1. Based on one recent meta-analysis (12), the average relative risks (RRs) and 95% confidence intervals (CI) for the highest vs lowest quantile of consumption of red meat were 1.35 (95% CI: 1.21~1.51) and of processed meat, 1.31 (95% CI: 1.13~1.51). Total meat consumption was not significantly associated with colorectal cancer risk. In terms of the measurable nutrients in meat, it is not clear which are truly associated with colorectal cancer risk. The Nurses??Health Study, prospective cohort study consisting of 120,000 registered nurses, showed that women in the highest quartile of red meat intake, compared with those in the lowest quartile, showed a 2.75-fold increase in colon cancer risk, even after adjusting for fat intake (13). Giovannucci and Gordin concluded that the association with red meat consumption does not appear to be mediated by its lipid content (14). Sugimura and Sato proposed that heterocyclic amines (HCAs), which are potent carcinogens, are associated with the risk of colon cancer (15). HCAs are formed by heating creatinine with amino acids, which occurs when meat is fried, grilled, or broiled at high temperature (16). Some studies have suggested, but not all (17), that risk of colon cancer (18,19) or adenoma (20) may be increased among those who prefer meat with browned surface. These observations raised the question as to whether enzymatic variabilities in the metabolism of heterocyclic amines, such as N-acetyltransferase (NAT), might influence the risk of colorectal neoplasia (21). Although no independent association with NAT1 (22) or NAT2 (23) genotypes were found, some studies (24~26) reported that rapid acetylators at either the NAT1 or NAT2 locus show a stronger association between red meat intake and colorectal cancer, suggesting that genetic polymorphisms may interact with meat consumption. Furthermore, the prevalence of the K-ras mutation, which is observed in 40 to 50% of colorectal neoplasm (27), has been closely linked to the rapid acetylator phenotype of the NAT2 enzyme. Therefore, NAT2 may determine genetic susceptibility to adenomas, carcinomas, and specific gene mutations, and may thus modify the association between meat consumption and colorectal cancer.
2) Vegetables, Folate, and Related Genetic Polymorphisms
Higher intakes of vegetables have been reported to be associated with a reduced risk of colorectal cancer (28,29). These effects of vegetables have been particularly consistent for raw vegetables, green vegetables, and cruciferous vegetables (8). It was suggested that the effect of vegetables on the risk of colon cancer appears to act through other pathways than dietary fiber (30). Folate, a water-soluble B vitamins, was suggested to be responsible for the beneficial effect of vegetables.
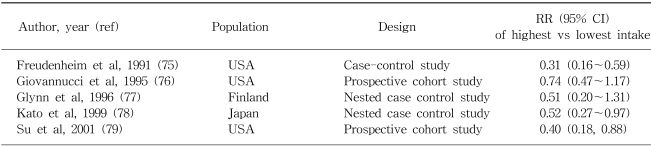
Epidemiologic studies on the effect of folate in colorectal cancer are limited. Lashner et al (31) first made a relevant observation in ulcerative colitis patients in a case-control study, they found that individuals who had not been regularly taking folate supplements had a rate of colonic neoplasms approximately 2.5-fold greater than those who had been taking supplements. Since then, fourteen studies have examined the association between folate intake and colorectal cancer risk and five studies have evaluated folate intake in relation to the risk of adenomatous polyps, the precusor lesions of colorectal cancer (32). However, results from case-control studies have been inconsistent. Inverse associations between higher intakes of folate and colorectal cancer risk have been more consistently observed in prospective studies. The effect estimates between folate and colon cancer for some selected populations are shown in Table 2.
Several mechanisms have been proposed for the effect of folate. First, folate is critical for the synthesis of S-adenosylmethionine (SAM), a compound that serves as an essential methyl donor for over 100 biochemical reactions, including the methylation of DNA (33). Consequently diminished folate intake might favor global and/or regional DNA hypomethylation, which appears to be an early, and consistent event in carcinogenesis (34). Second, the misincorporation of uracil into human DNA might be favored when thymidylate availability is restricted, i.e., due to folate deficiency (35,36), which is related to an increased frequency of chromosome cleavage (37). Finally the secondary depletion of choline due to folate deficiency might activate protein kinase C (PKC) signaling, which is related with mitogenesis and to the enhanced expression of the c-myc proto-oncogene (38). PKC activation has been reported to occur early in the development of chemically induced colonic neoplasia (39), and in human colorectal cancers (40).
Methylenetetrahydrofolate reductase (MTHFR) is a key regulator of the folate metabolism. MTHFR converts 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is the major form of folate in the blood and the primary methyl donor in the formation of methionine. A common genetic polymorphism of MTHFR (C677T, alanine-to-valine) has recently drawn much interest in the etiology of colorectal cancer. Individuals with a homozygous variant of the MTHFR (TT genotype) were found to have a reduced risk of colorectal cancer in several studies in the United States (41~44). This effect of the homozygous mutation of MTHFR was hypothesized to be related to the quantitative balance of 5, 10 methylenetetrahydrofolate and 5-methyltetrahydrofolate (41). However, in a study conducted in the United States (41,42), a protective association between the TT genotype and colorectal cancer was pronounced when folate or methionine intake was high and was disappeared when alcohol intake was high, suggesting that the effect of MTHFR genetic polymorphism is modified by the levels of dietary factors related with methyl metabolism. Contrary to these protective findings with TT genotype of MTHFR, an increased risk of CRC with the TT genotype was reported in some case-control studies conducted in Australia, especially among the elderly (45), and in Mexico (46). A similar positive association was also observed in a case-control study conducted in Korea (unpublished data). These findings raise the question as to whether the effect of MTHFR may differ according to the intake patterns of these dietary factors across countries.
3) Physical Activity, Obesity, and Related Mechanisms
The relation between physical activity and a reduced risk of colon cancer is among the most consistent findings in the epidemiologic literature. Based on one recent review (47), prospective and retrospective studies, upon occupational, leisure, and total activities, support an inverse association between physical activity and the risk of colon, but not of rectal cancer. Physically active men and women show a 30~40% dose-dependent reduction in the risk of colon cancer, compared with inactive individuals. The similar, but stronger risk reduction was also reported for the heavy leisure time physical activity among the elderly in a case-control study conducted in Korea (48). The effect estimates between physical activity and colon cancer for some selected populations are shown in Table 3. Obesity appears to be associated with an increased risk of colon cancer. For example, body mass index (BMI) was positively associated with colon cancer and large (≥ 1 cm) adenoma but not with small adenoma (49). When physical activity and obesity were assessed jointly, the highest risk of colon cancer was found among those who were both physically inactive and had high BMI levels (50,51).
Several biologic mechanisms have been proposed to explain the inverse association between physical activity, body mass, and colon cancer. One favored hypothesis concerns the relation between these factors and insulin. Hyperinsulinemia is related to physical inactivity and a high BMI (52). Moreover, insulin is mitogenic for normal and neoplastic colonic epithelial cells, and has been shown to be a colon tumor promoter in animal model (53). In addition, high insulin levels have been related prospectively to colon cancer risk (54). Hu et al (55) reported an increased incidence of colorectal cancer among diabetes mellitus patients in the Nurses' Health Study. It appears that insulin acts as a common denominator in colon cancer and other metabolic disorders, such as diabetes mellitus and dyslipidemia. Actually, hyperinsulinemia has been proposed to underlie the association between a Western lifestyle and colon cancer risk (56).
Insulin-like growth factor (IGF) axis influences cellular proliferation and apoptosis. Insulin-like growth factor-I (IGF-I) is a potent mitogen for normal and neoplastic cells, whereas IGF-binding protein-3 (IGFBP-3) has been found to inhibit cell growth in many experimental systems. Recently it was reported that colorectal cancer is positively related to plasma insulin-like growth factor I (IGF-I), and inversely related to insulin-like growth factor binding protein 3 (IGFBP-3) by several prospective studies (57~59). Further Ma et al (60) reported that elevated insulin production, as reflected by an elevated concentrations of plasma C-peptide, appeared to predict the risk of colorectal cancer development, independently of BMI, factors related to insulin resistance, or levels of IGF-I and IGFBP-3 in a nested case-control study. Recently a T-to-A polymorphism in the growth hormone (GH)1 at position 1663, which is supposedly conferring lower levels of IGF-1, was found to be associated with a decreased risk of colorectal cancer in a case-control study conducted in Hawaii (61). These associations were, however, observed in Caucasians and Native Hawaiians but not in Japanese.
4) Alcohol and Related Genetic Polymorphism
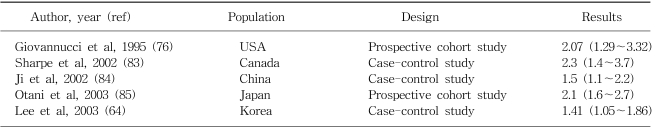
Alcohol intake has been related to an increased risk of colorectal cancer although the increase is relatively small (8). A meta-analysis of 5 cohort studies and 22 case-control studies published from 1966 to 1989 showed a weak positive association (62). In a recent pooled analysis of 8 prospective cohort studies from Western countries, an 16% increase in the risk of colorectal cancer was reported among those consuming 30g or more of alcohol per day, with no appreciable differences shown between colon and rectal cancers in this regard (63). Similar association was also reported in a case-control study conducted in Korea (64). No convincing evidence suggests that different sources of alcohol present different risk (65). The association is likely to be related to total ethanol intake, irrespective of the type of drink. The effect estimates between alcohol consumption and colon cancer for some selected populations are shown in Table 4.
Several mechanisms are conceivable with respect to the increased risk of colorectal cancer associated with alcohol intake. In rats fed a diet with a normal folate content, alcohol administration increased intracolonic acetaldehyde levels and significantly reduced colonic mucosal folate levels (66), possibly due to cleavage of folate by acetaldehyde (67). Elevated alcohol intake may also be related with delayed DNA repair, the activation of liver procarcinogen by the induction of cytochrome p-450 enzymes (68), or a change in bile acid composition (69). The beneficial effect of the MTHFR TT genotype on the risk of CRC was abolished in those with high alcohol intakes (41,42), suggesting a possible interaction between genotypes and folate metabolism. Further, the association with colon cancer due to alcohol consumption appeared to be pronounced among the heterozygotes of Aldehyde dehydrogenase 2 (ALDH2), which encodes a mitochondrial enzyme responsible for the oxidation of acetaldehyde generated in alcohol metabolism, compared with the wild type (70).
Summary
Based on current evidences, there appears to be more than one pathway to the development of colorectal cancer. Environmental factors, such as obesity, physical activity, folate, red meat consumption, and alcohol drinking, appear to interact with specific genetic polymorphisms to modify the risk of colon cancer (summarized in Table 5). Thus further studies incorporating genetic and environmental factors are needed to fully explain and characterize the nature of the pathway that underlies colorectal carcinogenesis.