The Relationship between Expression of the Sodium/iodide Symporter Gene and the Status of Hormonal Receptors in Human Breast Cancer Tissue
Article information
Abstract
Purpose
It has been reported that the sodium/iodide symporter (NIS) gene is expressed in several breast cancer tissues, suggesting the possibility of radionuclide imaging and therapy. However, the regulatory mechanism of NIS gene expression in breast cancer is not yet understood. To assess the relationship between the hormonal status and the NIS expression in breast cancer tissue, we investigated the NIS expression and correlated it to the expression of the thyrotropin receptor (thyroid stimulating hormone receptor, TSH-R), the estrogen receptor (ER) and the progesterone receptor (PR) in human breast cancer tissues.
Materials and Methods
Breast cancer tissues were obtained from 44 patients. Pathological examination showed 2 cases of Grade I, 17 of Grade II, 22 of Grade III, and 3 of unknown grade. We measured the expression of NIS and TSH-R genes by using RT-PCR and we measured the status of ER and PR by using immunohistochemistry.
Results
The NIS gene was expressed in 15 (34%) of the 44 breast cancer tissues. The NIS gene was expressed in 32% of the cases with TSH-R gene expression. The NIS gene was expressed in 40% of the breast cancer tissues with a positive PR and in 31% with a negative PR (p>0.05). It was positive for PR in 18% of the cases and negative for PR in 39% of the cases (p>0.05).
Conclusion
The NIS gene is expressed in approximately one-third of the human breast cancer tissues. Its expression was not related to the presence of the TSH-R gene or hormonal receptors, ER and PR.
INTRODUCTION
The sodium-iodide symporter (NIS) is an integral membrane protein of thyroid follicular cells. It transports active iodide necessary for thyroid hormone synthesis. Accordingly, radioiodines are used for the diagnosis and treatment of various thyroid diseases (1~4). Most differentiated thyroid carcinomas retain NIS protein activity. This provides the basis for the use of radioiodine in the detection and therapy of thyroid cancer.
Moreover, iodide also accumulates in the lactating mammary gland and is secreted into milk (5). Like thyroid cancers, it is possible that breast cancers express the NIS gene and protein. Several investigators have reported the expression of NIS protein and the NIS gene in various cases of human breast cancer (6~9). In addition, the scintigraphic visualization of breast cancer using 99mTc-pertechnetate, which is also transported by NIS, has been suggested as a new imaging modality (7).
However, the regulatory mechanism of NIS expression in breast cancer has not yet been established. Hormones may be important factors in the regulation of NIS expression and in the carcinogenesis of breast cancer. Although thyrotropin (thyroid stimulating hormone, TSH) is known to upregulate NIS expression in thyroid cells (5,8,10), its role in the regulation of NIS in breast cells remains controversial (8). Estrogen has also been thought to increase the level of NIS, because it has a direct effect on NIS transcription (11). Moreover, Tazebay et al. (5) showed that the combination of estrogen, prolactin, and oxytocin can increase the expression of NIS in ovariectomized mice.
We evaluated the NIS expression and compared it with the expression of the thyrotropin receptor (TSH-R), the estrogen receptor (ER) and the progesterone receptor (PR) in human breast cancer tissues.
MATERIALS AND METHODS
1) Tissue sample
Surgically removed breast cancer tissues were obtained from 44 female patients (age range: 30~74 years) with invasive ductal carcinoma. The samples were classified histologically according to the Nottingham modification of Bloom-Richardson and presented as Grade I (2 samples), Grade II (17 samples), Grade III (22 samples), and as unknown (3 samples).
2) RNA isolation and reverse transcription polymerase chain reaction
The tissues were frozen at -70℃ in isopentane and stored in liquid nitrogen before use. Total RNA was isolated using the Trizol agent/chloroform method (Invitrogen, Carlsberg, CA). Five µg of the purified RNA was reverse transcribed using 50 units of Superscript II (Invitrogen, Carlsberg, CA) in a 20µl reaction mix containing random primer or oligo (dT) primer.
3) Polymerase chain reaction
The quality of the cDNA, produced by RT-PCR, was determined by using PCR with the conditions as described below. Also used were a pair of primers from the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene sequence (sense, 5'-ACCAGGGCTGCTTTTAACTCT-3'; antisense, 5'-GAGTCCTTCCACGATACCAAAG-3'), which were predicted to amplify a 468 bp fragment.
The primer pairs selected (sense, 5'-GCTAAGTGGCTTCTGGGTTG-3'; antisense, 5'-GTAAGCACAGGCCAGGAAAA-3') for human NIS cDNA were used to amplify a 450bp fragment. The reaction profile consisted of 1 cycle at 94℃ for 5 min, followed by 30 amplification cycles of; 94℃ for 1 min, 52℃ for 30 s, and 72℃ for 10 min. PCR amplification of the TSH-R gene was also performed, using a pair of primers predicted to produce a 196 bp fragment (sense, 5'-GAAGCTGTACAACAACGGC-3'; antisense, 5'-TCAGTTCCTTCAGGTGCTCC-3'). The PCR reaction conditions involved 1 cycle at 94℃ for 5 min, followed by 30 amplification cycles of; 94℃ for 30 s, 53℃ for 30 s, and 72℃ for 7 min. After amplification, 20µl of the reaction mix was separated on a 2% agarose gel. The resulting bands were visualized under UV light by using ethidium bromide staining. In all experiments, water was used as the negative control.
4) Immunohistochemistry
Eight-micrometer frozen sections were obtained from tumor tissues and fixated with ethanol. These sections were incubated with each of the anti-ER and anti-PR antibodies for 2 h at 25℃. Rabbit polyclonal antibodies against ER and PR were purchased from Charles River Pharmservices (Southbridge, MA) and were diluted to 1:500 and 1:100, respectively, as was recommended by the manufacturer. Each section was stained for ER and PR using the avidin/biotin conjugate immunoperoxidase procedure and then incubated with the second antibody for 30 min at 25℃. After washing with phosphate-buffered saline for 15 min the sections were incubated with streptavidin peroxidase and washed with phosphate-buffered saline. 3,3'-Diaminobenzidine terahydrochloride was used as a substitute substrate-chromogen solution and sections were counter-stained with Meyer's hematoxylin. Adjacent sections incubated with excess rabbit immunoglobulin G were used as negative controls.
5) Statistical analysis
NIS and TSH-R expressions were correlated to their possible relation to the presence of ER and PR. Results were analyzed using the Mann-Whitney U-test and the chi square test.
RESULTS
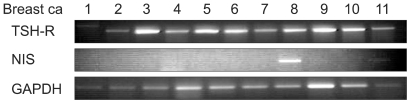
NIS mRNA was expressed in 15 (34%) of the 44 breast cancer tissues (Fig. 1) and the TSH-R gene was expressed in 34 cases (77%) (Fig. 1). Immunohistochemical staining for ER or PR were positive in 15 (34%), and 11 cases (25%), respectively.
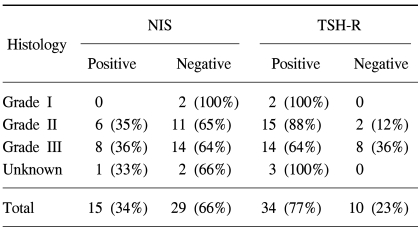
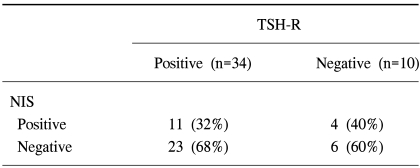
Table 1 represents the relations between NIS or TSH-R gene expression and the histological grade of breast cancer. The NIS gene was expressed in 6 cases (35%) of Grade II, 8 cases (36%) of Grade III, but was not expressed in Grade I. No statistical difference in NIS gene expression versus pathologic grade was observed. TSH-R was expressed in 2 cases of Grade I, 15 (88%) cases of Grade II, and 14 (65%) cases of grade III. Interestingly, breast cancers without TSH-R expression seemed to have higher pathologic grades than those with TSH-R expression (p<0.05). In addition, no correlation was found between the expression of NIS and TSH-R. Among the 34 cases positive for TSH-R, NIS was expressed in 11 cases (32%). The NIS gene was not expressed in 4 (40%) of the 10 cases, which did not express the TSH-R gene (Table 2).
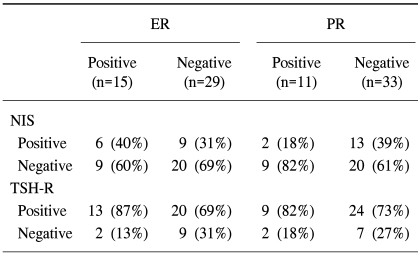
The expressions of NIS and TSH-R were not related to the expressions of ER and PR (Table 3). The NIS gene was expressed in 40% of the cases with positive ER, and in 31% with negative ER (p>0.05) and in 18% of the cases positive for PR and in 39% of the cases negative for PR (p>0.05). TSH-R was expressed in 13 (87%) of 15 positive ER samples, and in 20 (69%) of 29 negative ER samples (p>0.05). TSH-R was also expressed in 9 (82%) of the 11 positive PR cases, and in 24 (73%) of the 31 negative PR cases. There was no statistical relation between the menopausal status and NIS or TSH-R expression.
DISCUSSION
Several investigations have reported on NIS expression in breast cancer. Tazebay et al. (5) showed that the NIS gene is expressed in more than 80% of breast cancer tissues and Wapnir et al. (12) showed that 76% of invasive breast carcinomas react to NIS antibody staining positively. Similar findings have been reported by other investigators (7,8). In the present study, we found that the NIS gene was expressed in 34% of the 44 breast cancer tissues, which is somewhat lower than that of the previous reports. The discrepancy may be due to differences in the evaluation procedures, sample numbers, or race. For example, Moon et al. (7) found that only 4 of 25 Korean patients with breast cancer showed an accumulation of 99mTc pertechnetate in tumor tissue, while Upadhyay et al. (8) reported that almost all Indian patients showed positive tumor accumulations.
It is well known that NIS is expressed in the normal breast and that catalyzed iodide accumulates during lactation and gestation (5). NIS expression during lactation could be induced through hormonal regulation. Moreover, NIS expression was found to increase markedly after the administration of a combination of 17-beta-estradiol, oxytocin, and prolactin in combination. This effect was reduced in the presence of progesterone (13,14). Thus, it is possible that similar mechanisms of NIS expression exist in breast cancer and lactating breast tissues. Although many scientists have hypothesized that NIS expression may be affected by hormonal factors, this has not yet been confirmed.
In the thyroid, the expression of NIS is normally regulated by several factors, including TSH and cAMP. TSH upregulates NIS gene expression and increases radioiodide uptake by thyroid cancer cells and normal thyroid follicular cells (3,10,15). Furthermore, TSH is responsible for the translocation of NIS into the thyroid gland (16). In the absence of TSH, NIS is observed intracellularly, whereas in the hormone's presence, NIS was found to be targeted to the plasma membrane. Hence, TSH stimulates not only the transcription and biosynthesis of NIS, but also its retention at the plasma membrane of thyroid follicular cells. For these reasons serum TSH levels should be clinically elevated when 131I is used as a therapy in thyroid cancer.
This TSH effect is possible only in the presence of TSH-R in thyroid cancer cells and it has been reported that NIS expression is enhanced by TSH treatment in rat thyroid follicular cell (10). However, the role of TSH in the regulation of NIS in breast cells remains controversial (8,17~19). We investigated the expression of TSH-R in breast cancer tissues, and found no significant correlation between NIS expression and TSH-R expression even though the high frequency of TSH-R expression in breast cancer. Indeed the NIS proteins of thyroid cells and of lactating breast cells have a somewhat different structure. rNIS in the lactating mammary gland is less glycosylated than rNIS in the thyroid (14).
Furthermore, we found that NIS gene expression is not correlated with the level of other hormonal receptors such as ER and PR. Thus, hormones that regulate NIS expression in the lactating breast may not play a role in breast cancer. It is important to identify those factors required for NIS protein synthesis and activity in breast cancer.
Interesting, we found that TSH-R expression is correlated with tumor pathology type, as TSH-R gene expression more frequently observed in low-grade breast cancer. It had been thought that TSH-R and other genes are degenerated as the breast cancer progresses. However, we found no relationship between NIS expression and the pathologic grade.
There is a possibility of being able to use NIS and radionuclide for the detection and treatment of breast cancer. Moon et al. (7) and Upadhyay et al. (8) reported upon diagnostic scanning using 99mTc-pertechnetate. Moreover, 131I can be used to treat breast cancer, in similar fashion as it use in thyroid cancer (1). Dadachova et al. (20) suggested 188Re therapy instead of 131I, because they share the same NIS protein and because 188Re has better physical characteristics for radionuclide therapy. However, preliminary reports were not encouraging and they highlighted issues that remain to be resolved. The present study was undertaken for this very purpose. Our results suggest that TSH, estrogen, and progesterone play no role in improving NIS expression and the tumor accumulation of such radionuclides. In the future, the identification of the mechanisms and characteristics of NIS expression in breast cancer may provide new potential therapies and diagnostic tools for clinical application in human breast cancer.
CONCLUSIONS
In this study, NIS gene expression was found in approximately one-third of the 44 cases of human breast cancer tissues. In addition no relationship was found between the expression of NIS, TSH-R gene, and hormonal receptors, ER and PR gene.