Long-term Outcomes of Protocol-Based Treatment for Newly Diagnosed Medulloblastoma
Article information
Abstract
Purpose
The Korean Society of Pediatric Neuro-Oncology (KSPNO) conducted treatment strategies for children with medulloblastoma (MB) by using alkylating agents for maintenance chemotherapy or tandem high-dose chemotherapy (HDC) with autologous stem cell rescue (ASCR) according to the risk stratification. The purpose of the study was to assess treatment outcomes and complications based on risk-adapted treatment and HDC.
Materials and Methods
Fifty-nine patients diagnosed with MB were enrolled in this study. Patients in the standard-risk (SR) group received radiotherapy (RT) after surgery and chemotherapy using the KSPNO M051 regimen. Patients in the high-risk (HR) group received two and four chemotherapy cycles according to the KSPNO S081 protocol before and after reduced RT for age following surgery and two cycles of tandem HDC with ASCR consolidation treatment.
Results
In the SR group, 24 patients showed 5-year event-free survival (EFS) and overall survival (OS) estimates of 86.7% (95% confidence interval [CI], 73.6 to 100) and 95.8% (95% CI, 88.2 to 100), respectively. In the HR group, more infectious complications and mortality occurred during the second HDC than during the first. In the HR group, the 5-year EFS and OS estimates were 65.5% (95% CI, 51.4 to 83.4) and 72.3% (95% CI, 58.4 to 89.6), respectively.
Conclusion
High intensity of alkylating agents for SR resulted in similar outcomes but with a high incidence of hematologic toxicity. Tandem HDC with ASCR for HR induced favorable EFS and OS estimates compared to those reported previously. However, infectious complications and treatment-related mortalities suggest that a reduced chemotherapy dose is necessary, especially for the second HDC.
Introduction
Medulloblastoma (MB) is the most common malignant brain tumor in childhood, accounting for 10%-15% of all intracranial tumors [1,2]. Multimodality therapy, consisting of surgery, radiotherapy (RT), and chemotherapy, has been used to treat patients with MB. The survival rate with multimodality therapy is affected by age, metastatic stage at diagnosis, and resection status after surgery [3,4]. The revised 2021 classification system for central nervous system tumors defines MB according to histological and molecular types (i.e., wingless integrated [WNT], sonic hedgehog [SHH] with TP53-wildtype, SHH, and TP53-mutant, and non-WNT/non-SHH), which are associated with different genetics, clinical features, and prognosis [5].
Following the introduction of multi-agent chemotherapy for MB treatment in the 1970s, numerous studies have been conducted [6]. In a previous study, the standard-risk (SR) group was treated with 36 and 54 Gy cerebrospinal irradiation and total tumor bed /posterior fossa RT, respectively. However, adjuvant chemotherapy with 23.4 Gy of reduced cerebrospinal irradiation was recently attempted, and it achieved nearly 80% event-free survival (EFS) rate [7,8]. In the high-risk (HR) group, in the CCG 921 study conducted in the late 1980s, a 5-year EFS of approximately 40% was achieved with 36 and 55.8 Gy cerebrospinal irradiation and total RT, respectively [9].
Furthermore, CCG 921 was accompanied by adjuvant chemotherapy consisting of vincristine, 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU), and steroids [9]. Sandwich treatment with neoadjuvant chemotherapy before RT was administered in the PNET-3 and German HIT91 clinical trials and showed similar results [10,11]. High-dose chemotherapy (HDC) with autologous stem cell rescue (ASCR) transplantation is a treatment alternative and was shown to achieve a 5-year EFS of 70% [7].
To reduce long-term neurotoxicity associated with RT, dose-modified craniospinal irradiation (CSI) has been considered. The Children’s Oncology Group (COG) recommends lowering the radiation dose to the cerebrospinal axis by 25% for patients aged < 6 years. The attempt to reduce CSI from 36.0 Gy to 23.4 Gy with adjuvant chemotherapy in children with SR MB, showed similar survival outcomes along with improved cognitive outcomes [12]. A low-dose CSI of 18.0 Gy showed higher intelligence scores, although it did not improve the survival rate and showed inferior outcomes in patients in group 4 [13].
The increased survival rate of patients with MB has led to several late complications, such as neurocognitive function deterioration, hearing loss, and endocrine problems, which are considered important [14]. To prevent these complications, various treatment strategies that reduce the intensity of chemotherapy and RT or introduce modifications to the treatment method have been established. The Korean Society of Pediatric Neuro-oncology (KSPNO) developed protocols for MB in 2005 by using an alkylating agent after RT for SR MB patients and reduced-dose craniospinal RT (CSRT) and tandem HDC for HR patients [15].
Therefore, in this study, we aimed to analyze the treatment outcomes of MB patients, the complications during treatment, and the late effects to improve subsequent KSPNO protocols for MB.
Materials and Methods
1. Patients and risk groups
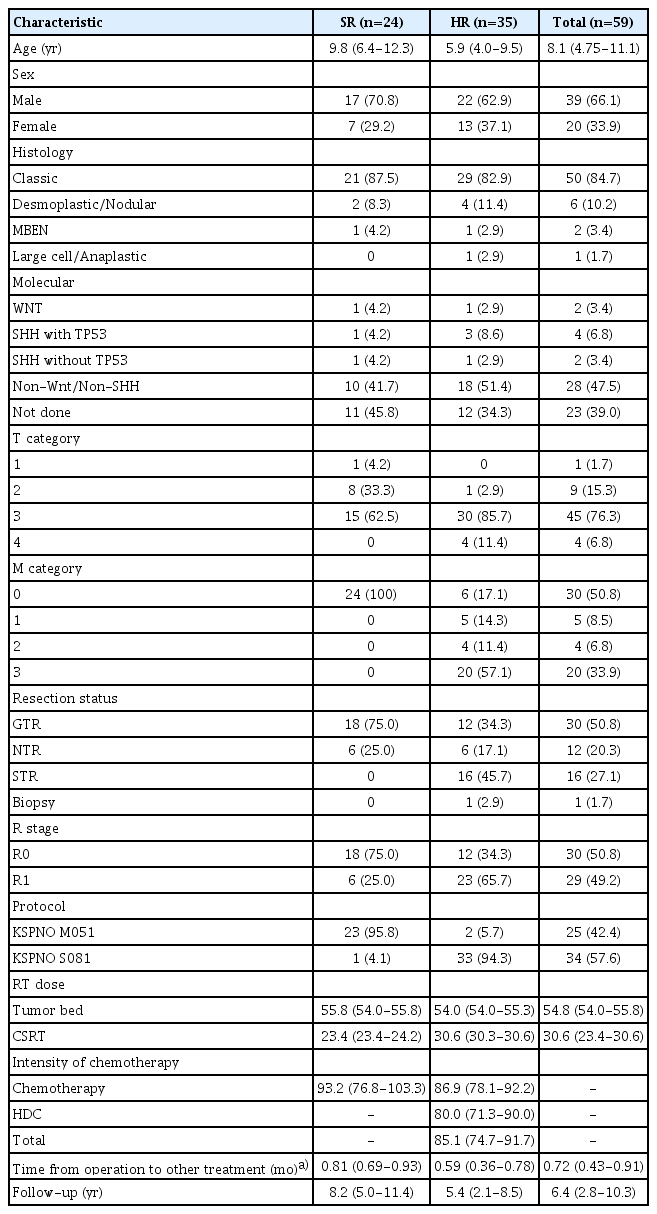
From April 2005 to March 2021, 86 patients were newly diagnosed with MB at Yonsei Cancer Center, Yonsei University Health System, Seoul, Korea. Twelve patients were < 3 years old. Five patients were treated with different treatment protocols, two discontinued the treatment protocol, three were transferred to other hospitals, and five refused chemotherapy. Fifty-nine of them treated with KSPNO M051 or S081 protocols, were retrospectively reviewed.
All diagnoses were confirmed and classified according to histological and molecular groups using immunohistochemical staining [16]. The KSPNO SR group was defined as patients with gross total resection (GTR) or nearly total resection (NTR) status and no metastasis at diagnosis. The molecular subgroup was determined according to the activation status of the WNT and SHH signaling pathways through analysis of immunohistochemical staining. MYC status was analyzed through fluorescence in situ hybridization. The HR group was defined as patients with residual tumors > 1.5 cm2 after surgery or metastasis at the time of diagnosis confirmed using cerebrospinal fluid (CSF) cytology or spinal magnetic resonance imaging (MRI). In total, 24 and 35 patients were included in the SR and HR groups, respectively.
At first diagnosis, brain and spinal MRI scans were performed to determine the stage and presence of metastatic lesions, including leptomeningeal seeding. Primary surgical resection was performed by qualified pediatric neurosurgeons at our institution. Furthermore, the extent of the resection was as much as possible according to the anatomical location of the tumor and considering the risk of the patient’s neurologic complications after surgery. The extent of resection was evaluated using an MRI scan taken within 48 hours after the operation.
2. Definition and risk stratification
Resection status was defined as GTR for no visible tumor after surgery, NTR for ≥ 95%, subtotal resection (STR; 50% to 95%), partial resection (10%-49%), and biopsy only for < 10%. The degree of residual disease (R stage) was defined as a negative margin of resection for R0 and a positive margin of resection or gross residual tumor for R1.
The metastatic stage (M stage) was determined through a CSF study using a lumbar puncture performed 7 days after surgery and a postoperative spinal MRI scan within 1 week of surgery. The M stages were described according to Chang’s staging system [1]. Briefly, in this system, M1, M2, M3, and M4 stages are described as microscopic tumor cells found in the CSF, intracranial nodular seeding, spinal nodular or seeding lesions identified using imaging studies, and extraneural metastasis, respectively.
3. Treatment
The KSPNO M051 regimen for the SR group consisted of surgery, followed by RT and chemotherapy. The children received eight cycles of cyclophosphamide, vincristine, and cisplatin and four cycles of cyclophosphamide and vincristine (Table 1, Fig. 1). RT comprised 23.4 Gy CSRT with a three-dimensional conformal boost to the tumor bed (55.8 Gy) with weekly vincristine 1.5 mg/m2 as a radiosensitizer.

The Korean Society of Pediatric Neuro-Oncology (KSPNO) protocol scheme for medulloblastoma. The KSPNO M051 protocol for the standard-risk group (A) and the KSPNO S081 protocol for the high-risk group (B). CSRT, craniospinal radiotherapy; CTx, chemotherapy; HD, high-dose; RTx, radiotherapy.
The KSPNO S081 regimen for the HR group consisted of chemotherapy before and after RT and tandem HDC with ASCR. Two cycles of chemotherapy were administered within 2-4 weeks of surgical resection; 23.4 Gy CSRT and 30.6 Gy tumor bed with weekly vincristine were administered to all patients with an M0 status. Treatment for the M1 stage differed according to age. RT was administered at the same CSRT dose to those with M0 status, boosted to the tumor with an additional 21.6 Gy for spinal seeding nodules in patients < 6 years old.
The older patients > 6 years old were administered 30.6 Gy for CSRT, 23.4 Gy for tumor bed and intracranial seeding nodules, and 14.4 Gy for spinal seeding nodules. Within 4 weeks of completing RT, four additional cycles of chemotherapy at a 75% reduced dose were administered to the patients. After completing six cycles of chemotherapy, tandem HDC with ASCR was administered for consolidation. For the first round of HDC, the carboplatin, thiotepa, and etoposide (CTE) regimen was used as a conditioning treatment, and the cyclophosphamide and melphalan (CM) regimen was used for the second round of HDC [17].
The period between the tandems was at least 12 weeks to reduce side effects [18]. Since 2015, the planned dose of HDC has been reduced to 80% owing to treatment-related mortality. Each chemotherapy course began when peripheral blood counts recovered to acceptable levels, with an absolute neutrophil count > 750/μL and platelet count > 75,000/μL.
4. Follow-up
After two to three chemotherapy cycles and before RT and HDC, an MRI scan was performed for disease status evaluation. Responses of MB and leptomeningeal seeding tumors were evaluated according to the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Criteria. In this assessment, complete response, partial response, and progression are defined as the disappearance of target lesions, a decrease of at least 30% in the sum of the diameters of the target lesions, and an increase at least 20% in the sum of diameters of the target lesions, respectively.
5. Toxicity
Treatment-related toxicities were monitored according to the Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0. All adverse events above grades 3, 4, and 5 that occurred during and after treatment were recorded.
6. Statistical analysis
Data are presented as median values with interquartile range (IQR), numbers with percentages, and means±standard deviation. The survival duration was calculated from the date of diagnosis to the last follow-up date. EFS was defined as the time from diagnosis to the first occurrence of death from any cause, relapse, progressive disease, or development of a secondary malignancy. Disease-specific survival (DSS) was defined as disease recurrence and death. Overall survival (OS) was defined as the time from diagnosis to death from any cause. Hazard ratios with associated 95% confidence intervals (CI) and p-values comparing outcome distributions were calculated using Cox regression.
For analyzing 5-year EFS, DSS, and OS, we focused specifically on patients in the SR treated with KSPNO M051 and in the HR treated with KSPNO S081. The Kaplan-Meier method was employed to analyze survival outcomes, and the log-rank test was used to assess statistical significance. Fisher’s exact test was used to analyze the parametric variables, and the Mann-Whitney U test was used for non-parametric variables. All statistical analyses were performed using the IBM Statistical Package for the Social Sciences (SPSS) ver. 23.0 (IBM SPSS Statistics, IBM Corp., Armonk, NY) and R statistical software ver. 4.1.0 (Foundation for Statistical Computing, Vienna, Austria).
Results
Fifty-nine patients aged > 3 years were enrolled and treated using the KSPNO protocol at Yonsei Cancer Center, Yonsei University Health System, Seoul, Korea. Table 2 shows their disease characteristics according to the SR and HR groups. The histologic diagnosis showed that classic MB was the most common type occurring in 50 patients (84.7%), followed by desmoplastic/nodular, medulloblastoma with extensive nodularity, and large cell/anaplastic types in six (10.2%), two (3.4%), and one (1.7%) patient, respectively. Twenty-three patients (95.8%) in the SR were treated with the KSPNO M051 protocol, and only one patient was treated with KSPNO S081 as recommended by the surgeon after total resection although there was no metastatic lesion on cytology or imaging study. Thirty-three patients (94.3%) in the HR were treated by KSPNO S081 protocol, and two patients were treated with KSPNO M051, respectively, due to delayed subsequential treatment from postoperative infection and parental refusal of HDC with ASCR.
1. Metastasis and resection status
In this cohort, 29 of 59 patients (49.2%) had metastases, consisting of five (8.5 %), four (6.8%), and 20 patients (33.9%) with M1, M2, and M3 metastases, respectively. Resection was performed where possible, and total or NTR was performed in 24 patients (100%) with SR and 18 of 33 (54.3%) with HR.
2. Radiotherapy
All patients were treated with RT with local radiation and CSRT. Patients in the SR group were administered 23.4 Gy (IQR, 23.4 to 24.2) of CSRT with a boost of 32.4 Gy (IQR, 30.6 to 32.4) to the tumor bed, whereas the HR group received 30.6 Gy (IQR, 30.3 to 30.6) of CSRT with a 23.4 Gy (IQR, 23.4 to 25.6) boost to the tumor bed and 14.4 Gy to the spinal seeding nodule.
3. Chemotherapy
The intensities of the chemotherapy doses were adjusted according to the patients’ general conditions and the level of bone marrow recovery. Considering the actual dose and duration of treatments, patients in the SR group received 93.2% (76.8%-103.3%) of the planned chemotherapy dose. Patients in the HR group received 86.9% (78.1%-92.2%) of the planned dose for induction chemotherapy, 80.0% (71.3%-90.0%) of the planned tandem HDC, and 85.1% (74.7%-91.7%) of the total intensity of the KSPNO S081 regimen. Furthermore, the period before RT or chemotherapy after surgery was 0.81 (0.69-0.93) and 0.59 (0.36-0.78) months in the SR and HR groups, respectively.
4. Survival outcome
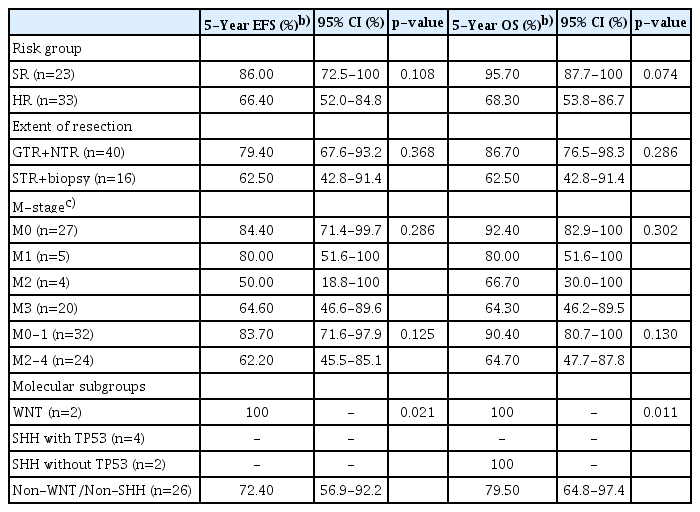
The median follow-up time was 8.2 (5.0-11.4) and 5.4 (2.1-8.5) years in the SR and HR groups, respectively. The estimated 5-year EFS and OS were 86.0% (95% CI, 72.5 to 100) and 95.7% (95% CI, 87.7 to 100) in the SR group and 66.4% (95% CI, 52.0 to 84.8) and 68.3% (95% CI, 53.8 to 86.7) in the HR group (Fig. 2A and B). Moreover, the estimated 10-year EFS and OS were 77.4% (95% CI, 59.2 to 100) and 82.2% (95% CI, 65.3 to 100) in the SR group and 62.5% (95% CI, 47.6 to 82.0) and 62.6% (95% CI, 46.7 to 84.0) in the HR group. There was a similar EFS and OS rate in patients with GTR or NTR and those with STR or biopsy only (p=0.4).
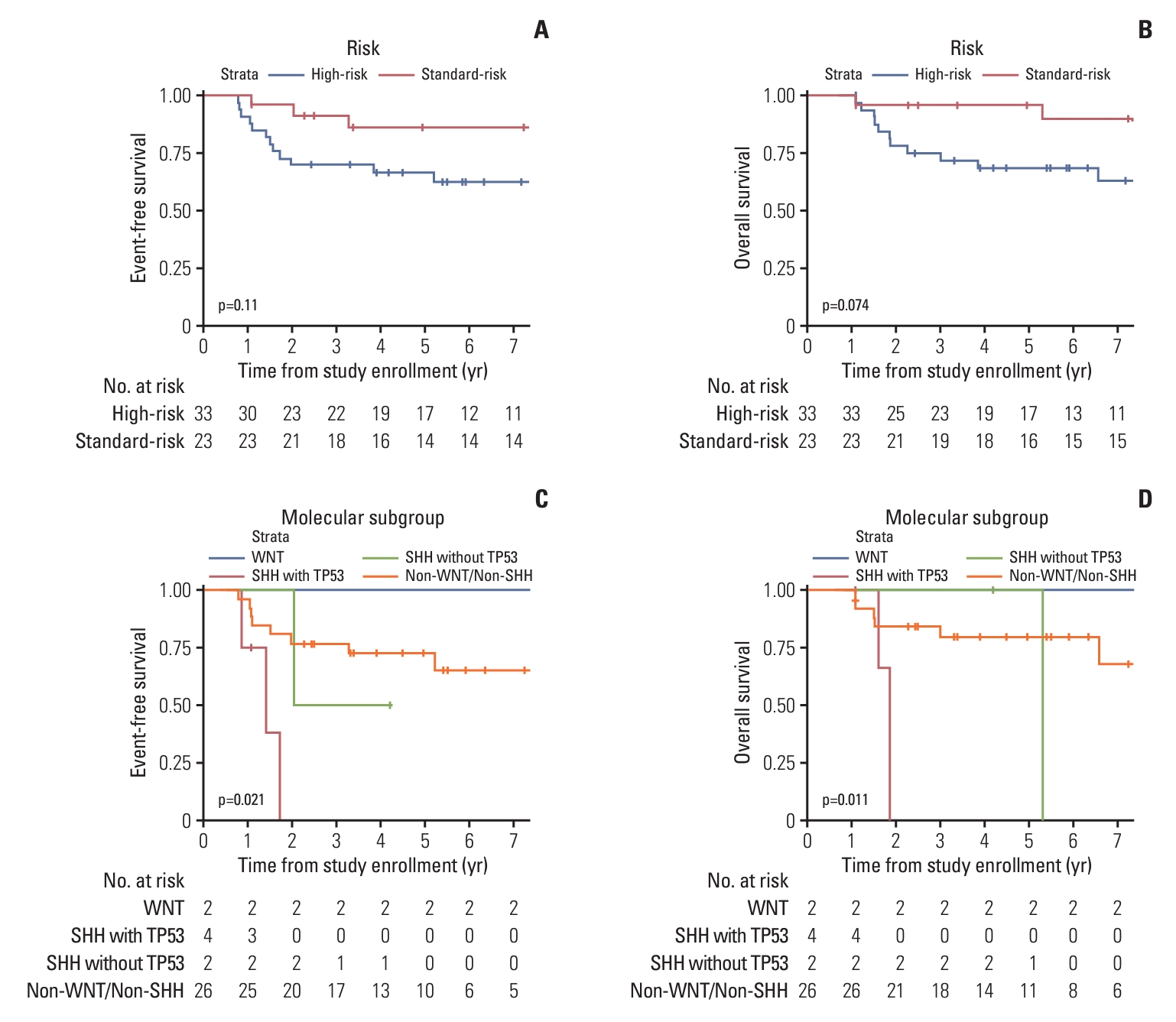
Event-free survival and overall survival for patients according to the risk group (A, B) and molecular subgroup (C, D).
The comparison according to metastatic status showed that disease progression and number of deaths were higher in the M2 and M3 groups than they were in the M0 and M1 groups, but the difference was not statistically significant (Table 3). Furthermore, the molecular subgroup analyses of 36 patients using immunohistochemical staining showed that patients with SHH with TP53 mutation had inferior EFS and OS (Fig. 2C and D). The Cox multivariate regression analyses performed with age at diagnosis, sex, residual tumor > 1.5 cm2 after surgery, metastatic status, intensity of chemotherapy, molecular subgroup, and duration from surgery to subsequent treatment showed no significant differences.
5. Progression or relapse during therapy
There were 10 cases of tumor recurrence and progression. In the SR group, two patients (8.3%) had progression of leptomeningeal seeding within 3 years of their diagnosis and subsequently died as a result. Three of the 17 patients who underwent STR or biopsy had local relapses. In the HR group, eight (22.9%) patients had progression or relapse, consisting of four each with local relapse and leptomeningeal seeding, whereas seven of them consequently died. In addition, one patient treated with the KSPNO M051 regimen developed a secondary malignant neoplasm (undifferentiated pleomorphic sarcoma) in the occipital lobe and skull within the radiation field 9 years after his diagnosis. He was treated with resection of the tumor and adjuvant chemotherapy and is now alive without evidence of disease 12 months after diagnosis of a second malignancy.
6. Outcomes according to chemotherapy intensity
In the SR group, seven patients who received chemotherapy were administered ≤ 80% of the planned dose, and two of them experienced disease progression and subsequently died 5 years after diagnosis (p=0.041). In the HR group, 30 patients were treated with HDC, including 26 who completed tandem HDC. There was no difference in DSS and OS between patients who received > 80% and those who received < 80% of the total planned treatment dose (Table 4).
7. Treatment toxicity and patient mortality
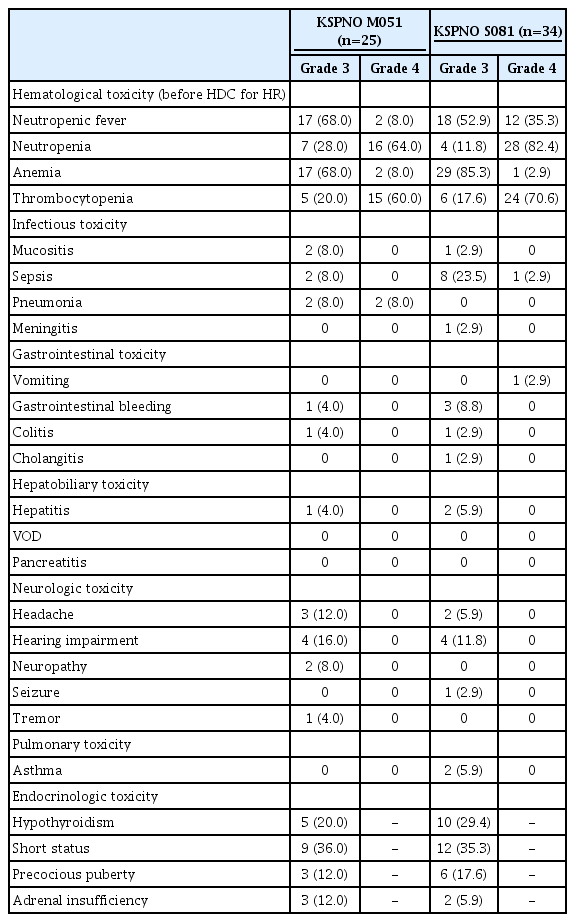
Toxicities of ≥ grade 3 according to the CTCAE that occurred during chemotherapy and HDC with ASCR are summarized in Tables 5 and 6, respectively. In the KSPNO M051 regimen, hematologic and infectious toxicities of ≥ grade 3 were the most common. When administering the KSPNO M051 regimen, two patients (8.0%) had treatment-related mortalities, one died of cytomegaloviral pneumonitis in the SR, and the other died of pneumonia and acute respiratory distress syndrome in the HR.
When bone marrow recovery was delayed, the intensity of the regimen was reduced by extending the interval between chemotherapy cycles or individual modification of the planned dose in the KSPNO M051 regimen for SR patients. However, two patients (SHH without TP53, one patient; non-WNT/non-SHH, one patient) treated with ≤ 80% of the planned dose experienced recurrence and died. The 10-year progression-free survival (PFS) and DSS of patients administered > 80% of the planned dose were both 100%, whereas values of those who received ≤ 80% of the planned dose were 68.6% (p=0.03) and 60.0% (p=0.04), respectively (S1 Table). However, one patient (WNT subgroup) of > 80% of the planned dose experienced secondary malignancy within the RT field 9 years after his diagnosis.
Nine patients (26.5%) treated with the KSPNO S081 regimen before HDC had sepsis, including one who had meningitis without mortality. During tandem HDC with ASCR, acute toxicities were mainly reported for infectious diseases and were more frequent in the second HDC (Table 6). Conversely, hepatitis was more frequent in the first HDC treatment group. Hepatic veno-occlusive disease (VOD) occurred in one (3.2%) and two (7.41%) patients during the first and second HDC, respectively. One patient died of myelopathy after the first HDC with ASCR, and three patients died during the second HDC with ASCR, consisting of one each of VOD with hepatorenal syndrome, pneumonia with acute respiratory distress syndrome, and uncontrolled gastrointestinal bleeding.
Discussion
In this cohort study of patients with MB over 3 years of age, chemotherapy and RT administered according to the KSPNO protocol showed favorable treatment outcomes. In the SR, survival outcomes are better than those reported in other studies [7,13,19-21]. However, the HR group showed toxicity to be overcome, despite similar survival outcomes as in previous studies [3,7,22,23].
In the previously conducted COG A9961 and SIOP PNET-4 studies (S2 Table), patients with MB in the SR group who received CSRT (23.4 Gy) and RT boost to the tumor bed (55.8 Gy) with various combinations of chemotherapy showed 80% 5-year EFS [19-21]. In the SJMB 96 and 03 studies, patients with MB in the SR group received the same dose of RT with HDC plus ASCR four times every 4 weeks. The results showed a similar EFS of 83% without treatment-related mortality [7]. In the COG ACNS 0331 study, reducing RT to 18 Gy for CSRT in young children showed inferior outcomes [13].
In the KSPNO M051 protocol, patients received the same dose of RT and chemotherapy as that of the higher-intensity alkylating drug, instead of CCNU. The total intensity was higher than that of the total HDC in the SJMB 96 study, and the total duration of chemotherapy was longer at 48 weeks than that in the COGA9961 study, where a similar weekly average intensity of chemotherapy was used.
Survival outcomes were promising, but frequent hematologic and infectious toxicities occurred in the KSPNO M051 protocol. A dose reduction below 80% of the planned dose showed a risk of relapse. Based on this finding, we believe that the RT and planned dose intensity of chemotherapy should be maintained to minimize recurrence; however, it has to be considered individually with the molecular subgroup and the risk of treatment-related mortality, as also noted in this cohort.
To increase the survival rate of MB patients with HR, various attempts have been made to modify postoperative RT from 36 Gy of CSRT and to change the combination and intensity of chemotherapy (S3 Table). In the GPOH-HIT study, neoadjuvant and adjuvant chemotherapy with RT and combinations of various chemotherapies were used, and the 5-year EFS was > 60% [11,22]. In the SIOP PENT-3 protocol, RT alone was compared with RT and adjuvant chemotherapy, and the combination showed better results than RT alone [10]. Maintenance therapy with alkylating agents has been used in an attempt to improve the survival rate of metastatic patients [3,23]. Hypofractionated radiation therapy (HFRT) was used in the COG and HIT studies, but did not show better survival outcomes than previous regimens; therefore, a follow-up study is ongoing [22,24].
In addition, HDC for HR MB has been considered part of the treatment for patients in the HR or relapsed state [7,25]. In the SJMB study, 36 Gy CSRT and HDC with cyclophosphamide, cisplatin, and vincristine with ASCR were administered four times every 4 weeks, resulting in an approximately 70% 5-year EFS without treatment-related mortality [7,26]. In the Milan study, two cycles of HDC were administered according to the response to pre-HFRT, which showed similar outcomes [27]. In the PNET HR+5 studies, two cycles of high-dose thiotepa with ASCR showed 76% of 5-year PFS without treatment-related mortality [28]. In relapsed patients, HDC with thiotepa, busulfan, and melphalan for myeloablation and RT could be salvaging treatments for newly relapsed patients [29].
In this study, two and four cycles of chemotherapy were administered before and after lower RT for patients in the HR, respectively. Furthermore, chemotherapy was administered based on the higher total intensity of alkylating drugs such as cyclophosphamide, ifosfamide, and platinum analogs than those used in previous studies. To compensate for using a lower RT intensity than that used in previous studies, we administered tandem HDC with ASCR for HR patients [15]. Two cycles of tandem HDC were included in the treatment course for consolidation, with an interval of at least 12 weeks to reduce complications from HDC [18]. Compared to the studies on SJMB, the number of HDC with ASCR was lower, however, the whole intensity of the conditioning chemotherapy was higher in this study [26]. The 5-year EFS and OS rates in the HR group in our study were similar to those observed in studies of standard CSRT without HDC [3,22,23] or lower total dose of HDC [7,26,27].
In fact, in HR patients treated with the KSPNO S081 regimen, there was no difference in the 5-year DSS and OS between patients with a dose modification of less than 80% and patients in the planned dose group (S3 Table). Treatment-related mortalities occurred frequently during the second HDC with ASCR until 2014, when the planned HDC dose was reduced. The 5-year OS of the group administered > 80% dose intensity was 60%, and the 5-year DSS of this group was 15% higher than the 5-year OS. Moreover, there was a higher 5-year OS tendency in the group administered ≤ 80% of the HDC dose intensity than there was in the other group. Consequently, a dose intensity reduction of tandem HDC with ASCR might be reasonable.
A large national database analysis confirmed that patients who underwent STR or biopsy did not have inferior survival outcomes to those who underwent GTR or NTR. In this study, there was no significant difference in the outcomes between the two groups [30]. This observation indicates that insufficient surgical resection could be overcome by additional RT for residual tumors. Patients with a metastatic status are treated with chemotherapy before RT, HDC with ASCR, and maintenance chemotherapy, but this strategy is consistently associated with an inferior outcome [13,22]. We attempted to overcome this inadequate response by using a higher-intensity alkylating agent and tandem HDC with ASCR, but it did not show superior outcomes to those of previous studies.
Molecular subgroup genetic and methylation analyses are an important strategy for determining survival outcomes, and the clinical characteristics of each group have also been analyzed [31,32]. In addition to the previously known molecular subgroups, such as WNT, SHH, group 3, and group 4, a more subdivided classification is suggested to improve disease risk stratification and find a better treatment strategy [24]. At the time these protocols were developed, the molecular subgroup had not been incorporated, although the subgroup was analyzed using immunohistochemical staining. However, WNT subgroups showed superior outcomes, whereas those with SHH with TP53 mutation had inferior outcomes, similar to previously reported findings [32].
Some limitations of this study need to be addressed. First, risk stratification was based on age, residual disease, and metastatic lesions as conventional clinical parameters. Second, the old specimens from the resection of the tumor were not classified according to the revised World Health Organization (WHO) histological classification. Molecular subgroups were only classified in half and only for WNT and SHH with or without TP53 mutation. Finally, the number of enrolled patients was small, and the comparison of the treatment’s effectiveness was difficult because of a single-armed and single-center study. Despite these limitations, this cohort underwent treatment using a unified protocol. Treatment with the KSPNO protocol resulted in excellent survival outcomes in children with MB. KSPNO plans to develop a future protocol for risk stratification with molecular subgroups and risk-adapted treatment. Designing the future protocol by adjusting RT according to the risk group and appropriate HDC with ASCR, may be key for further studies.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the Institutional Review Board (IRB) of Yonsei University Health System Clinical Trials Center (IRB No. 4-2022-0634). Informed consent was waived because of the retrospective analysis of anonymous clinical data.
Author Contributions
Conceived and designed the analysis: Kim DS, Suh CO, Lyu CJ, Han JW.
Collected the data: Ahn WK, Lee J, Kim SH, Han JW.
Contributed data or analysis tools: Ahn WK, Hahn SM, Yoon HI, Lee J, Park EK, Shim KW, Kim DS, Suh CO, Kim SH, Lyu CJ, Han JW.
Performed the analysis: Ahn WK, Kim SH, Han JW.
Wrote the paper: Ahn WK, Han JW.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.