Pembrolizumab for Patients with Relapsed or Refractory Extranodal NK/T-Cell Lymphoma in Korea
Article information
Abstract
Purpose
Programmed death-1 blockade with pembrolizumab has shown promising activity in relapsed/refractory (R/R) extranodal natural killer/T-cell lymphoma (NKTCL), but studies are limited, with small patient numbers.
Materials and Methods
Thirteen institutes involved with the Consortium for Improving Survival of Lymphoma, a Korean lymphoma study group, collected the clinical data of 59 patients treated with pembrolizumab as salvage therapy between 2016 and 2022.
Results
The median age of the patients was 60 years (range, 22 to 87 years), and 76.3% had advanced Ann Abor stage disease. Pembrolizumab was given to 35.6%, 40.7%, and 23.7% of the patients as second-, third-, and fourth- or higher-line chemotherapy, respectively. The overall response rate was 40.7%, with 28.8% having complete response. The estimated 2-year progression-free survival (PFS) and overall survival rates for all patients were 21.5% and 28.7%, respectively; for responders, the rates were 53.0% and 60.7%, respectively. Although not statistically significant, Eastern Cooperative Oncology Group performance status ≥ 2 (hazard ratio [HR], 1.91; 95% confidence interval [95% CI], 0.93 to 3.94; p=0.078) and stage III or IV disease (HR, 2.59; 95% CI, 0.96 to 6.96; p=0.060) were associated with a trend toward shorter PFS in multivariate analysis. Grade 3 or 4 adverse events (AEs) were noted in 12 patients (20.3%); neutropenia (10.2%), fatigue (6.8%), and pneumonitis (5.1%) were most common AEs.
Conclusion
In conclusion, while pembrolizumab had a modest effect on patients with R/R NKTCL, it may be a useful salvage therapy for patients with localized disease and good performance status.
Introduction
Extranodal natural killer/T-cell lymphoma (NKTCL) accounts for 5%-10% of non-Hodgkin lymphomas in Asia and Central America but is uncommon in Western nations [1]. In more than two-thirds of NKTCL cases, the disease localized at the time of diagnosis [2]. For first-line treatment of localized nasal NKTCL, the combination of nonanthracycline-based chemotherapy with radiation therapy has favorable prognosis, with a 5-year progression-free survival (PFS) rate of 73% to 89% [3,4]. On the other hand, treatment outcomes for advanced NKTCL are unsatisfactory, even with L-asparaginase–containing therapies, and the prognosis for relapsed/refractory (R/R) NKTCL is dismal [5,6].
Recent advances in molecular biology and genetics have opened new avenues for NKTCL pathobiology and potential therapeutic targets [7,8]. Programmed cell death-ligand 1 (PD-L1) and programmed cell death-1 (PD-1) are key immune checkpoint molecules implicated in immune evasion [9]. Anti–PD1/PD-L1 antibodies are now among the most recommended anticancer treatments [10]. PD-L1 expression in tumor cells was observed to range from 56% to 93% in NKTCL studies [8,11-13]. PD-L1 positivity in tumor cells and immune cells invading the tumor is associated with improved overall survival (OS) [11,12]. There are recent case series demonstrating the effectiveness of PD-1 blockade with pembrolizumab, with a complete response (CR) rate ranging from 29% to 71%; however, response to PD-1 blockade remains equivocal because of small sample sizes [14-16]. Biomarkers for predicting the response of NKTCL patients to immune checkpoint inhibitors (ICIs) have not been identified. In this context, we report the largest study evaluating the efficacy and safety of pembrolizumab, in 59 patients, for R/R NKTCL from 13 tertiary institutes in Korea.
Materials and Methods
1. Study design and patients
We conducted a retrospective analysis to evaluate the efficacy and safety of pembrolizumab treatment in patients with R/R NKTCL. The clinical information of 59 patients treated with pembrolizumab as salvage therapy between September 2016 and June 2022 was collected from 13 institutions affiliated with the Consortium for Improving Survival of Lymphoma, a Korean lymphoma research group. Age, sex, Ann Arbor stage, Eastern Cooperative Oncology Group (ECOG) performance status, B symptoms, peripheral blood Epstein-Barr virus (EBV) DNA status, and treatment outcomes were obtained from patient medical records. Circulating EBV DNA was measured by quantitative polymerase chain reaction, and any measurable concentration of EBV DNA was considered positive.
2. Treatment and response evaluation
Doses of pembrolizumab were not uniform among participants in this study. Thirty-five patients (59.3%) were dosed at 2 mg/kg, and five patients (8.5%) were dosed at a fixed dose of 200 mg every 3 weeks. The remaining 19 patients (32.2%) received a lower fixed dose of 100 mg every 3 weeks due to the cost burden of pembrolizumab, which is not covered by health insurance in Korea. Pembrolizumab was administered, which continued until disease progression, unacceptable toxicity, or patient refusal. Response was evaluated in accordance with the recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma (the Lugano classification) by using 18F-fluorodeoxyglucose positron emission tomography integrated with computed tomography and computed tomography scans [17]. Adverse events (AEs) were assessed throughout the study duration according to National Cancer Institute Common Terminology Criteria for Adverse Events (ver. 4.03).
3. Outcomes
The primary endpoint was the CR rate, which was based on the best response over the course of the treatment; a secondary endpoint was the overall response rate (ORR), which was defined as the proportion of all enrolled patients who achieved either CR or partial response (PR). Key secondary efficacy endpoints included PFS and OS. PFS was measured from the first administration of pembrolizumab to the date of disease progression, death from any cause, or last follow-up, whichever occurred first. OS was measured from the first administration of pembrolizumab to death from any cause or the end of the last follow-up, whichever occurred first. Safety profiles were among the additional secondary endpoints.
4. Statistical analysis
Using the Kaplan-Meier method, survival curves were produced and compared using the log-rank test. Prognostic variables for PFS and OS were identified by using the Cox proportional hazards model. Appropriate variables were compared between two groups using the χ2 test. All tests were two-tailed, and a p-value of < 0.05 was considered statistically significant. All statistical analyses were conducted using ver. 18.0 of the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL).
Results
1. Patient characteristics
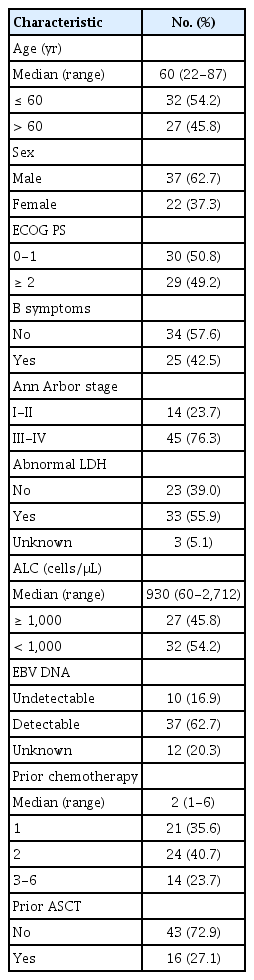
Table 1 presents the baseline characteristics of the 59 enrolled patients. The median age was 60 years (range, 22 to 87 years), and 37 patients (62.7%) were male. Approximately half of the patients (n=30, 50.8%) had ECOG performance status 0-1. At the time of enrollment, the majority of patients (n=45, 76.3%) were in stages III-IV of the disease. The presence of EBV DNA was assessed in 47 patients, of whom 37 (78.7%) had a detectable viral DNA titer based on each institute’s reference value. Pembrolizumab was given as second-, third-, and fourth-line therapy or more in 21 (35.6%), 24 (40.7%), and 14 (23.7%) patients, respectively. Prior to receiving pembrolizumab, 86.4% of patients were treated with L-asparaginase–containing chemotherapeutic regimens, and 26.2% had undergone autologous stem cell transplant.
2. Treatment and clinical outcomes
Pembrolizumab was delivered in a median of two cycles (range, 1 to 24). Reasons for discontinuation were because of progressive disease (PD) (n=35, 59.3%), CR (n=8, 13.6%), patient withdrawal (n=5, 8.5%), follow-up loss (n=4, 6.8%), physician decision (n=3, 5.1%), or toxicity (n=1, 1.7%). Three deaths (5.1%) occurred, one each from pneumonia, traumatic brain hemorrhage, and gastrointestinal bleeding due to disease; all were considered unrelated to pembrolizumab treatment.
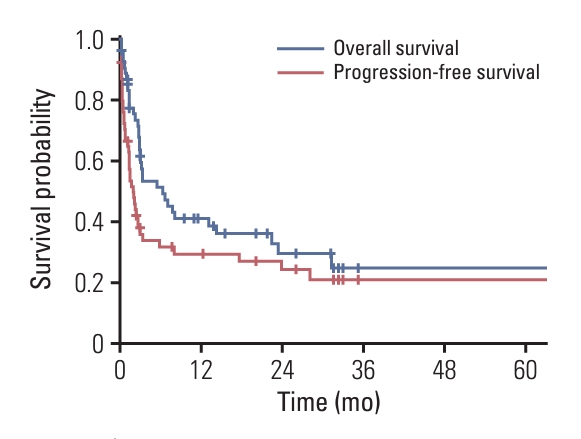
With an ORR of 40.7%, CR and PR were observed in 17 (28.8%) and seven (11.9%) patients, respectively. Patients with confirmed CR as best response, 10 (58.8%) remained in CR after a median follow-up of 29 months from pembrolizumab. Of all patients, 32 had PD as best response; three patients were unable to be evaluated for response. The survival curves of all patients are shown in Fig. 1. With a median follow-up of 3.2 months (range, 0.1 to 70.5 months), the median PFS and OS were 1.9 months (95% CI, 1.0 to 2.9) and 2.5 months (95% CI, 1.8 to 11.5), respectively. The estimated 2-year PFS and OS rates were 21.5% and 28.7%, respectively. According to response, there were statistically significant differences in PFS and OS (p < 0.001 and p < 0.001, respectively) (Fig. 2). After a median follow-up of 20.4 months (range, 1.0 to 70.5 months) for responders, the median PFS and OS were 24.4 months (95% CI, 15.2 to 33.6) and not reached (NR) (95% CI, NR to NR) for responding patients, respectively. The estimated 2-year PFS and OS rates for responders were 53.0% and 60.7%, respectively. In addition, the response was exceptionally durable, with the longest duration exceeding 78 months.
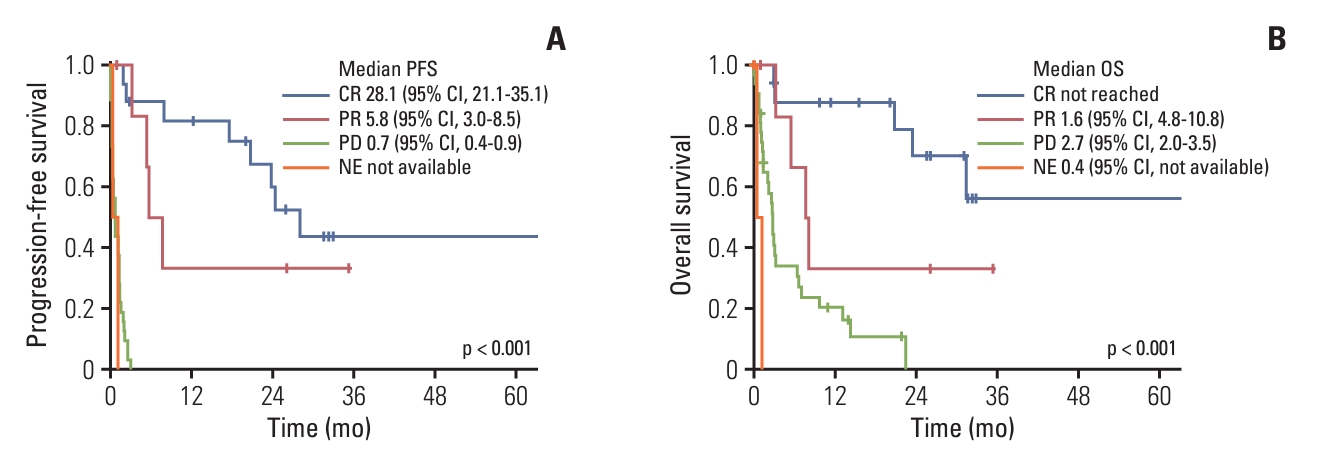
Survival curves according to response: progression-free survival (PFS) (A) and overall survival (OS) (B). CI, confidence interval; CR, complete response; NE, not evaluated; PD, progressive disease; PR, partial response.
Univariate analysis of all patients showed that ECOG performance status (PS) ≥ 2, B symptoms, absolute lymphocyte count (ALC) < 1,000 cells/μL and disease stage III or IV were significant predictors of PFS and OS (Table 2). The number of prior lines of therapy was not associated with PFS or OS. Prior to pembrolizumab treatment, a detection of EBV DNA in the blood was not associated with prognosis. Although not statistically significant, ECOG PS ≥ 2 (hazard ratio [HR], 1.91; 95% CI, 0.93 to 3.94; p=0.078) and stage III or IV disease (HR, 2.59; 95% CI, 0.96 to 6.96; p=0.060) were associated with a trend toward a shorter PFS in multivariate analysis. In multivariate analysis of OS, ECOG PS ≥ 2 (HR, 3.23; 95% CI, 1.49 to 7.00; p=0.003) and stage III or IV disease (HR, 5.13; 95% CI, 1.10 to 23.83; p=0.037) were statistically significant predictors of poor prognosis.

Univariate and multivariate analyses of PFS and OS in patients with ENKTL treated with the pembrolizumab
Of 43 patients who developed relapse or refractory disease after pembrolizumab treatment, 26 patients (60.5%) received various subsequent chemotherapy regimens. The majority of patients (22/26, 84.6%) were treated for pembrolizumab-refractory disease and the remaining patients (4/26, 15.4%) were treated for the disease relapse after pembrolizumab response.
3. Adverse events
Pembrolizumab treatment was well tolerated with no reports of treatment-related deaths. Grade 3 or 4 AEs were noted in 12 patients (20.3%); neutropenia (n=6, 10.2%), fatigue (n=4, 6.8%), and pneumonitis (n=3, 5.1%) were most common (Table 3). One patient received unrelated allogeneic stem cell transplantation 6 months before pembrolizumab. The patient received one dose of pembrolizumab but died due to disease progression; graft-versus-host disease was not evident.
Discussion
The prognosis for patients with R/R NKTCL is dismal, and their treatment represents an urgent unmet need. Herein, we describe the efficacy, safety, and predictive biomarkers of the largest study of pembrolizumab in patients with R/R NKTCL to date.
EBV-infected tumor cells frequently express PD-L1, binding of which to PD-1 on T cells transduces inhibitory signals, resulting in immune escape of tumor cells; it is postulated that EBV-infected tumor cells are sensitive to PD-1 inhibition [18]. Not all patients, however, respond to pembrolizumab. The CR and ORR for the patient cohort in the current study were 29% and 41%, respectively, which is comparable to the results of the most recent phase 2 trial of avelumab for this patient population (CR 24%, ORR 38%) [19]. Due to frequent early progression, patients had a median PFS of only 1.9 months, but those who responded to pembrolizumab demonstrated sustained antitumor activity. The estimated PFS and OS rates at 2 years for responders were 53.0% and 60.7%, respectively. Huang et al. [20] also demonstrated the efficacy of sugemalimab, an anti–PD-L1 antibody, in 80 patients with R/R NKTCL, achieving an ORR of 45% and a CR rate of 36%. Although the variation in response rate in these studies may be attributed to small sample sizes and patient heterogeneity, the results demonstrate that ICIs are efficacious in a subset of patients with R/R NKTCL. Consequently, the selection of NKTCL patients with a high likelihood of responding to ICIs may be crucial for enhancing the efficacy of ICIs.
PD-L1, as measured by immunohistochemistry, is the most validated, utilized, and recognized biomarker for selecting patients to receive anti–PD-1 or anti–PD-L1 antibodies in solid tumors [21]. There is a need for the development of biomarkers that can predict lymphoma treatment response to ICIs. Increased PD-L1/L2 protein expression in classical Hodgkin lymphoma (cHL) and primary mediastinal B-cell lymphoma (PMBCL) is indicative of a long PFS [22]. However, the clinical utility of PD-L1 testing for lymphomas other than cHL and PMBCL is unknown. In NKTCL patients, there is no strong correlation between expression of PD-L1 and therapeutic response [16,23]. Lim et al. [23] discovered a significant relationship between PD-L1 mutation and pembrolizumab responsiveness in patients with R/R NKTCL. The current understanding of the immune phenotype (IP) is determined by the status of tumor-infiltrating lymphocytes in the tumor microenvironment (TME), which comprises inflamed, immune-excluded, and immune-desert cells [24,25]. Clinical outcomes of ICIs have been described according to IP [26]. Cho et al. [27] demonstrated that TME subgroups identified by FoxP3, PD-L1, and CD68 staining as immune tolerance, immune evasion-A/B, and immune silenced are potential biomarkers for predicting ICI response. Kim et al. [19] found that PD-L1 expression in the tumor area was much higher in responders than in nonresponders and that immune subtyping correlated closely with response to avelumab. Immune phenotypes are currently quantified manually across whole-slide images, which restricts their applicability, impartiality, and reproducibility in routine clinical practice [28,29]. AI-powered spatial analysis of IP was found to correlate with tumor response and PFS with ICIs in advanced non–small cell lung cancer, demonstrating the therapeutic applicability of IP as a biomarker of ICIs [30].
The need for sufficient tumor tissue, the need to sequence DNA extracted from tumors, and the lack of standardized quantitative scoring systems for IP are limitations of these biomarkers that have impeded their widespread clinical application. There is a clinical need for biomarkers that may be quickly and affordably obtained in a variety of settings, including those with limited resources. Although not statistically significant, poor performance status and advanced staging tended to be associated with poor prognosis for ICI treatment, indicating prognostic and predictive relevance in the context. Valero et al. [31] found statistically significant relationships between the pretreatment neutrophil-to-lymphocyte (NLR) ratio and OS, PFS, and response in a large number of patients with various cancer types. In the present study, univariate analysis revealed that ALC was a significant predictor of PFS and OS but that NLR was not (data not shown).
This study has a number of limitations. Due to its retrospective nature, there may be undefined bias affecting clinical outcomes, and the results should be interpreted with caution due to the small sample size. However, to our knowledge, this is the largest retrospective study to evaluate the efficacy and safety of an ICI treatment in R/R NKTCL to date. Biomarker analysis of PD-L1 expression was unavailable due to a paucity of patient samples and the inaccessibility of samples from deceased patients. Doses of pembrolizumab were not uniform among participants in this study. Due to the small number of patients, interpretation of dose-related efficacy and safety analyses is limited, but there were no significant clinical benefit or safety differences between the standard dose group (2 mg/kg or 200 mg fixed dose) and the low dose group (100 mg fixed dose).
In conclusion, pembrolizumab had a modest effect on patients with R/R NKTL, but it may be an effective salvage therapy for patients with localized disease and good performance status. Additional work is necessary to identify potential response biomarkers and incorporate pembrolizumab into combination regimens with chemotherapy and/or immunotherapy agents.
Notes
Ethical Statement
The study was conducted in accordance with the Declaration of Helsinki and with the approval of the Institutional Review Board (IRB) at Seoul National University Bundang Hospital (B-2205-756104). The IRB waived the requirement for patient consent due to the retrospective nature of the investigation. Each participating institution’s institutional review board also approved the study.
Author Contributions
Conceived and designed the analysis: Lee JY, Lee JO.
Data collection and interpretation: Lee JY, Lee JO.
Contributed data: Lee JY, Kwon JH, Hur JY, Yi JH, Lee JH, Cho H, Do YR, Jo JC, Kang HJ, Koh Y, Lee WS, Lim SN, Yoon SE, Kim SJ, Lee JO.
Performed the analysis: Lee JY, Lee JO.
Wrote the paper: Lee JY, Lee JO.
Supervision: Lee JO.
Review and editing: Lee JY, Kwon JH, Hur JY, Yi JH, Lee JH, Cho H, Do YR, Jo JC, Kang HJ, Koh Y, Lee WS, Lim SN, Yoon SE, Kim SJ, Lee JO.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
The Lymphoma Working Group of the Korean Society of Hematology and the Consortium for Improving Survival of Lymphoma conducted this study. This research was supported by the Seoul National University Bundang Hospital Research Fund (09-20210001).