The Real-World Outcome of First Line Atezolizumab in Extensive-Stage Small Cell Lung Cancer: A Multicenter Prospective Cohort Study
Article information
Abstract
Purpose
The addition of immune checkpoint inhibitors to chemotherapy has improved survival outcomes in patients with extensive-stage small cell lung cancer (ES-SCLC). However, their real-world effectiveness remains unknown. Therefore, we investigated the effectiveness of atezolizumab plus chemotherapy in ES-SCLC in actual clinical settings.
Materials and Methods
In this multicenter prospective cohort study, patients with ES-SCLC receiving or scheduled to receive atezolizumab in combination with etoposide and carboplatin were enrolled between June 2021 and August 2022. The primary outcomes were progression-free survival (PFS) and the 1-year overall survival (OS) rate.
Results
A total of 100 patients with ES-SCLC were enrolled from seven centers. Median age was 69 years, and 6% had an Eastern Cooperative Oncology Group performance status (ECOG PS) ≥ 2. The median PFS was 6.0 months, the 1-year OS rate was 62.2%, and the median OS was 13.5 months. An ECOG PS of 2-3 and progressive disease as the best response were poor prognostic factors for PFS, while an ECOG PS of 2-3 and brain metastasis were associated with poor prognosis for OS. In addition, consolidative thoracic radiotherapy was found to be an independent favorable prognostic factor for OS (hazard ratio, 0.336; p=0.021). Grade ≥ 3 treatment-related adverse events were observed in 7% of patients, with treatment-related deaths occurring in 2% of patients.
Conclusion
We provided evidence of the favorable real-world effectiveness and safety of atezolizumab plus chemotherapy in ES-SCLC patients, including in the elderly and those with poor ECOG PS. Additional consolidative thoracic radiotherapy may also benefit ES-SCLC patients.
Introduction
Small cell lung cancer (SCLC) accounts for approximately 10%-15% of all lung cancers and shows rapid progression and poor prognosis relative to non-SCLC [1-3]. Etoposide-containing platinum-based chemotherapy has shown high response rates for extensive-stage (ES) SCLC [4-7]. However, despite high efficacy at the onset of administration, this effect is not sustained, and most patients with ES-SCLC experience disease progression, with a median overall survival (OS) of around 8-12 months [8,9].
Meanwhile, the high mutation rate associated with SCLC indicates its immunogenic nature, which is suggestive of potential responsiveness to immune checkpoint inhibitors [10,11]. Moreover, due to these SCLC characteristics, the addition of immune checkpoint inhibitors to chemotherapeutic treatments has been found to result in improved survival outcomes [9,12,13]. In 2018, the IMpower133 study investigated the efficacy of atezolizumab in combination with etoposide and carboplatin by comparing it to a placebo that was combined with etoposide and carboplatin [9]. The atezolizumab plus etoposide and carboplatin group demonstrated significant improvements in median OS (i.e., 12.3 vs. 10.3 months, p=0.007) and progression-free survival (PFS) (i.e., 5.2 vs. 4.3 months, p=0.02). Based on these results, the combination of four cycles of atezolizumab and platinum-based etoposide induction therapy, followed by atezolizumab maintenance therapy, has become the current standard first-line treatment for ES-SCLC [14].
However, to date few studies have evaluated the effectiveness of atezolizumab in actual clinical practice since the original clinical trial, and all of these were retrospective studies [15-17]. Therefore, in this prospective study, we aimed to investigate the real-world effectiveness of atezolizumab plus chemotherapy for ES-SCLC patients.
Materials and Methods
1. Patient inclusion criteria and study design
This study is a multicenter prospective cohort study in South Korea designed to investigate the real-world clinical effectiveness of atezolizumab combined with chemotherapy in patients with ES-SCLC. Prospective enrollment included adult patients aged over 18 who were histologically diagnosed with ES-SCLC at the initial diagnosis and had either initiated or were currently receiving atezolizumab from June 2021 to August 2022. The only exclusion criterion was that patients had already discontinued atezolizumab administration.
2. Treatment and assessment
Patients received atezolizumab in combination with etoposide and carboplatin for four cycles every three weeks according to the prior clinical trial regimen [9]. In addition to this initial therapy, they also received atezolizumab maintenance every 3 weeks until disease progression. Effectiveness evaluation was conducted every 6 weeks using computed tomography scans as per the recommendation of the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 for tumor assessments. PFS was defined as the time from the first day of treatment to disease progression or death from any cause, while OS was defined as the time from the first day of treatment to death from any cause. Adverse events (AEs) were identified via evaluation of vital signs, physical examination, and laboratory data according to the Common Terminology Criteria for Adverse Events ver. 5.0. The laboratory tests were performed before each treatment cycle, and additional tests were conducted at the investigator’s discretion.
3. Outcomes
The primary outcomes measured were the PFS and one-year OS rates of patients with ES-SCLC receiving atezolizumab plus chemotherapy. Secondary outcomes included effectiveness including OS, objective response rate (ORR), disease control rate (DCR), and safety.
4. Statistical analysis
Data collected from medical records were summarized using descriptive statistics, including mean±standard deviation, median (interquartile range [IQR]), and number (%). PFS and OS were analyzed using Kaplan-Meier survival curves and log-rank tests. Finally, Cox regression was used to calculate the hazard ratios of variables predicting PFS and OS.
PFS or OS were defined as the period from the initiation of atezolizumab administration until either disease progression or death, respectively. DCR was defined as the percentage of patients who were determined to have shown a complete response (CR), a partial response (PR), or stable disease (SD) based on radiological review at any of the participating institutions following the RECIST ver. 1.1 guidelines. ORR was defined as the percentage of patients with either CR or PR. A p-value of less than 0.05 was considered to be statistically significant. All statistical analyses were performed using IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY).
Results
1. Patients and baseline characteristics
From June 2021 to August 2022, a total of 100 patients with ES-SCLC from seven centers were eligible. After considering exclusion criteria, all 100 patients were enrolled in the study (Fig. 1). The median age of the patients was 69 years; with patients aged 75 or over accounting for 25% of the study population (Table 1). Of all patients, 92% were male, and 6% had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 2 or above. At the time of diagnosis, 26% of patients had brain metastasis. Of these, 19 patients received treatment for brain metastasis, and among the patients without brain metastases, three patients received prophylactic cranial irradiation during the maintenance phase. At the data cutoff point (i.e., 10 July 2023), the median follow-up period for patients was 13.2 months (IQR, 6.6 to 22.4). Ninety-one patients completed four cycles of atezolizumab plus chemotherapy, and a median of three cycles of atezolizumab maintenance therapy (IQR, 0 to 7) were administered. Subsequently, 52 patients received subsequent treatment (S1 Table).
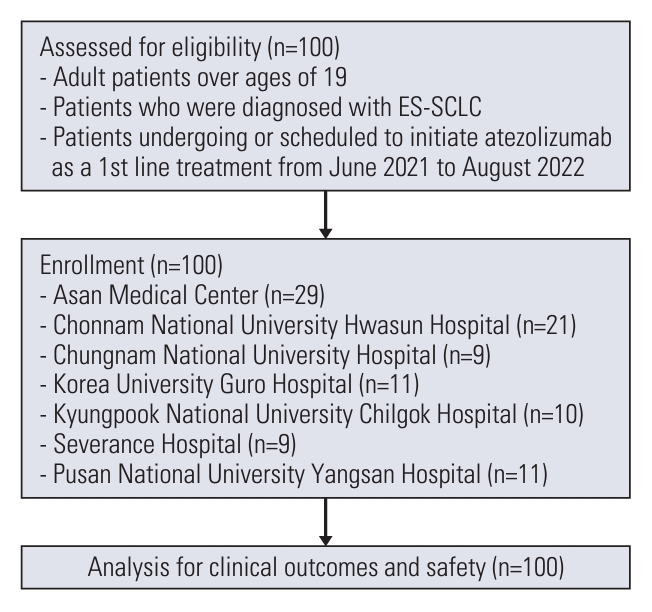
Flowchart of patients with small cell lung cancer receiving atezolizumab plus chemotherapy. ES-SCLC, extensive-stage small cell lung cancer.
2. Assessment of PFS, OS, and effectiveness
By the data cutoff, disease progression had occurred in 78 patients including 12 with intracranial-only progression, and the median PFS was 6.0 months (IQR, 4.2 to 11.0). Moreover, the 6- and 12-month PFS rates were 50.2% and 18.0%, respectively (Fig. 2A). On the other hand, 41 patients had died by the data cutoff, and the median OS was 13.5 months (IQR, 7.8 to not reached). The 6- and 12-month OS rates of this group were 85.8% and 57.8%, respectively (Fig. 2B).

Progression-free survival (PFS) (A) and overall survival (OS) (B) for all patients with small cell lung cancer receiving atezolizumab plus chemotherapy. IQR, interquartile range; N/E, not evaluable.
Regarding the best response, 67.0% of the patients showed a PR, and 15.0% of the patients had SD (Table 2). Moreover, the ORR was 67.0% and the DCR was 82.0%.
3. Prognostic factors
The results of our multivariable Cox analysis showed that both an ECOG PS of 2 or 3, and a best response of progressive disease (PD) were identified as poor prognostic factors for PFS (Table 3). Meanwhile, an ECOG PS of 2 or 3, and brain metastasis at diagnosis were found to be poor prognostic factors for OS, whereas consolidative thoracic radiation therapy (RT) was favorable (hazard ratio [HR], 0.371; 95% confidence interval [CI], 0.165 to 0.833; p=0.016).
A total of 26 patients received consolidative thoracic RT, and the clinical outcomes related to RT are represented in S2 Table and S3 Fig. Among those who had brain metastasis at the time of diagnosis, 19 patients received treatment for brain metastasis (stereotactic radiosurgery, 13 patients; whole brain radiation therapy, 6 patients). The survival curves according to brain metastasis and its treatment are shown in S4 and S5 Figs.
4. Safety
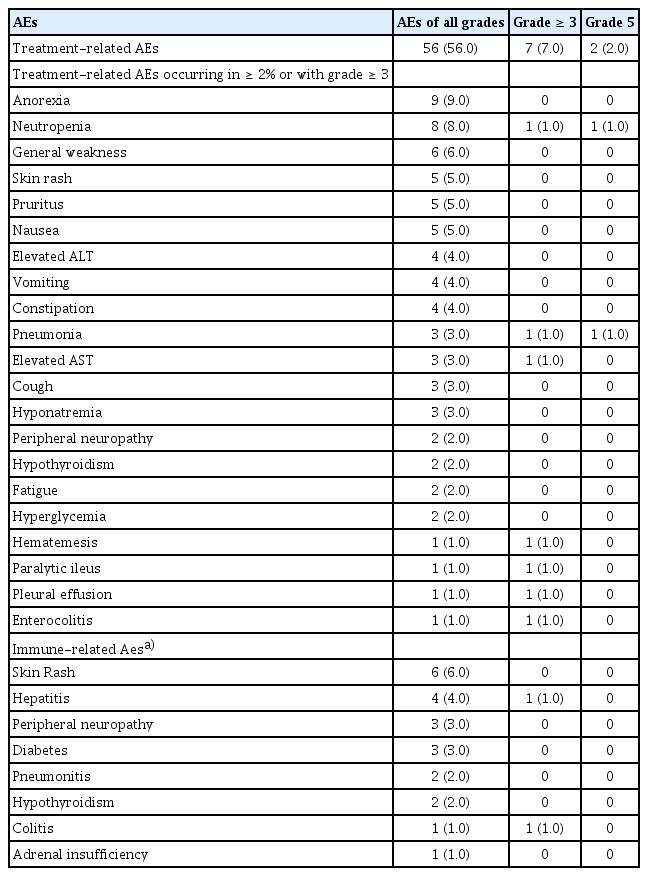
Next, safety assessments were conducted for all 100 patients (Table 4). Treatment-related AEs were observed in 56 patients, with anorexia being the most common. Neutropenia, general weakness, skin rash, pruritus, and nausea were the next most frequently reported AEs. Grade 3 or higher AEs occurred in 7% of patients, and we observed two treatment-related deaths, including one case of pneumonia, and one case of neutropenia.
Discussion
This study was the first multicenter prospective study conducted in a real-world clinical setting to evaluate the effectiveness and safety of first-line atezolizumab plus chemotherapy in patients with ES-SCLC. Considering the outcomes of the IMpower133 study (median PFS, 12.3 months; median OS, 12.3 months; OS rate at 1-year, 51.7%), the results of this study, with a median PFS of 6.0 months, a median OS of 13.5 months, and a 1-year OS rate of 57.8%, also showed favorable outcomes. An ECOG PS of 2-3 and PD as the best response were identified as poor prognostic factors for PFS, while an ECOG PS of 2-3 and brain metastasis at diagnosis were associated with poor prognosis for OS. In addition, consolidative thoracic RT was an independent favorable prognostic factor for OS. Finally, grade 3 or higher treatment-related AEs were observed in 7% of patients, with treatment-related deaths occurred in 2% of patients.
To the best of our knowledge, following the publication of the IMpower133 study by Horn et al. in 2018 [9], only a few retrospective studies investigated the outcomes of atezolizumab plus chemotherapy in real-world settings [15-17]. For example, Elegbede et al. [17] retrospectively evaluated the efficacy of atezolizumab plus chemotherapy in 34 patients with ES-SCLC, and reported a median PFS of 6.0 months and an OS of 12.8 months. Similarly, Sagie et al. [16] conducted a retrospective study of 54 patients with ES-SCLC treated with atezolizumab plus chemotherapy and reported a median OS of 353 days. More recently, Kim et al. [15] performed a retrospective study of 41 patients with ES-SCLC receiving atezolizumab, and found a median PFS of 5.1 months and a median OS of 15.2 months. The patient characteristics in these studies were heterogeneous, reflecting actual clinical practice. Moreover, the proportion of patients with an ECOG PS of 2 or higher ranged from 17% to 24%, and the median age—ranging from 65 to 67—was slightly higher than previous clinical trial. Furthermore, these studies also reported that an ECOG PS > 2, age, high lactate dehydrogenase levels, M1c stage, and a lack of thoracic radiation treatment were poor prognostic factors for measures of patient survival. However, these studies were limited by small sample sizes and the nature of retrospective studies.
Compared to the IMpower133 study where the median age was 64 years, our study had a slightly older median age of 69 years, with 25% of patients included over 75 years old. Moreover, we had six patients with an ECOG PS of 2 or higher. The rate of brain metastasis in our study was also considerably higher, i.e., ~25% or almost three times the previous study. Interestingly, despite having higher prevalence of factors associated with poor prognosis, including age, ECOG PS, and brain metastasis [18-21], the PFS and OS in this study were also favorable, although a statistical comparison was not feasible. We propose several hypotheses that may explain these results.
The first hypothesis to account for these results is that outcome improvements reflect the implementation of consolidative thoracic RT, which was not permitted in the IMpower133 study. In our study, 26% of patients underwent consolidative thoracic RT at the discretion of physicians, which was initiated approximately 3.8 months (IQR, 3.0 to 4.8) after the onset of atezolizumab treatment. Although this intervention did not show a statistically significant improvement in PFS (HR, 0.659; 95% CI, 0.380 to 1.143; p=0.138), it was an independent and statistically significant prognostic factor of improved OS (HR, 0.371; 95% CI, 0.165 to 0.833; p=0.016). Moreover, when comparing survival curves to those of consolidative RT, patients who underwent RT appeared to have favorable outcomes for around 1-year before the two groups’ survival curves converged. Moreover, although the follow-up duration was not sufficient, the OS curves of the two groups seemed to separate and the RT group appeared to show better survival outcomes than the non-RT group (S2 Table, S3 Fig.). This suggests that RT may delay disease progression in the early phases of treatment. However, additional follow-up for further validation would be needed to substantiate this hypothesis.
To date, the benefits of thoracic consolidative RT have been demonstrated by various studies [22,23]. For example, Slotman et al. [22] studied 247 patients with ES-SCLC who underwent thoracic consolidative RT and found that they showed a significantly higher 2-year OS rate compared to a control group (i.e., 13% vs. 3%, p=0.004). Moreover, in this study RT was a favorable prognostic factor for PFS (HR, 0.73; 95% CI, 0.61 to 0.87; p=0.001) [22]. Furthermore, Rathod et al. [23] recently used a meta-analysis study to show that consolidative thoracic RT significantly improved PFS (HR, 0.72; 95% CI, 0.61 to 0.83; p < 0.001). Similar improvements in OS were also observed in retrospective studies of atezolizumab plus chemotherapy in combination with the implementation of thoracic consolidative RT (HR, 0.33 to 0.44) [15,17]. There are several hypotheses related to the mechanistic basis of the benefits of RT. For instance, thoracic consolidative RT was found to significantly lower the intrathoracic recurrence rate by almost 50% compared to a control group in a phase 3 randomized controlled trial (p < 0.0001), which suggests that local-regional control is an important predictor of clinical outcomes [22]. Tang et al. [24] also hypothesized that localized radiation causes tumor-antigen release, which augments the antitumor immune responses of immune checkpoint inhibitors. Our study is consistent with the view that consolidative thoracic RT is beneficial.
Another potential explanation for the favorable performance observed even in this real-world study may be related to demographic characteristics. In the IMpower133 study, Asians accounted for 16.4% of patients studied, while in our study all patients were Asian. According to a retrospective study conducted by Ou et al. [25], being Asian was an independent prognostic factor for OS in a multivariate analysis of 4,782 patients with ES-SCLC (vs. Caucasian: HR, 0.785; 95% CI, 0.657 to 0.938; p=0.0076). Thus, the outcome of this current study may also be attributed to differences in ethnic composition.
Next, we note that the safety profile in this current study was also acceptable. Treatment-related deaths were similar to those in previous clinical study (2% vs. 1.5%), but the overall AEs, especially AEs of grade 3 or higher, were minimal (7% vs. 58.1%). However, we must be cautious in interpreting results related to safety. Even when including grade 1-2 AEs, the study showed a lower percentage compared to previous clinical study (56% vs. 94.9%). Several potential reasons account for this. One is the temporal factor associated with the coronavirus disease 2019 (COVID-19) pandemic. This study was conducted during the COVID-19 pandemic, which could have reduced patient visits and increased administrative burdens, potentially influencing the lower AE reporting rate [26-28]. Another study also showed that the frequency of severe AE reporting was significantly lower for patients enrolled after the pandemic compared to those enrolled before [28]. Another reason might be the characteristics of the real-world study setting. In such studies, AEs may not be as rigorously monitored as in clinical trials, with analysis potentially relying primarily on investigator reports. Consequently, mild symptoms, or those deemed insignificant in relation to chemotherapy by either patients or investigators, might have been overlooked. Furthermore, the patients analyzed in this study had various comorbidities and were administered chemotherapy along with other medications. This might have obscured the correlation between AEs and chemotherapy, leading to potential underreporting of AEs.
This study has several limitations. The first is that our patient sample included only Asian subjects, and therefore may not provide a fully representative view of the global patient population. However, this study is the first to report real-world outcomes, and it may therefore serve as evidence for future international studies. The second limitation is that we were unable to verify differences in outcomes related to various biomarkers, including programmed death-ligand 1, and drive-gene mutation in tissue. In the real world, most tissues are obtained through endobronchial ultrasound-guided transbronchial needle aspiration, percutaneous needle aspiration, or bronchoscopic biopsy. Thus, in most cases, there is insufficient tissue for additional molecular testing. Therefore, to confirm the correlation between tissue biomarkers and outcomes, considerations related to tissue acquisition for molecular testing should be included in the original study design. Last, the follow-up duration was insufficient to verify long-term survival. Nevertheless, we were able to confirm the benefit of atezolizumab plus chemotherapy for PFS and OS even in a real-world population. Moreover, we were also able to identify favorable prognostic factors of consolidative thoracic RT for OS. Currently, 17 patients are still undergoing atezolizumab maintenance, and their OS could therefore be updated.
In conclusion, this study is the first real-world prospective cohort study to provide evidence regarding the favorable effectiveness of atezolizumab plus chemotherapy for ESSCLC patients, including elderly patients or those with poor ECOG PS. Treatment-related AEs were acceptable even in the real-world setting. In addition, we suggest that consolidative thoracic RT may be beneficial during the atezolizumab maintenance phase when the disease is controlled.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study was approved by the relevant institutional review board This prospective cohort study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2021-1237) and was registered by the clinical research information service (Registration No. KCT0006818). Informed consent was obtained from all participants prior to enrollment. Finally, the trial was designed and conducted in accordance with the Helsinki Declaration and the Ethical Guidelines for Clinical Studies.
Author Contributions
Conceived and designed the analysis: Choi MG, Kim YJ, Lee JC, Ji W, Oh IJ, Lee SY, Yoon SH, Lee SY, Lee JE, Kim EY, Choi CM.
Collected the data: Choi MG, Choi CM.
Contributed data or analysis tools: Choi MG, Kim YJ, Lee JC, Ji W, Oh IJ, Lee SY, Yoon SH, Lee SY, Lee JE, Kim EY, Choi CM.
Performed the analysis: Choi MG, Choi CM.
Wrote the paper: Choi MG, Choi CM.
Supervision: Lee JC, Choi CM.
Writing - review and editing: Choi MG, Kim YJ, Lee JC, Ji W, Oh IJ, Lee SY, Yoon SH, Lee SY, Lee JE, Kim EY, Choi CM.
Conflicts of Interest
I-JO received grant from Roche. I-JO received personal fee from Gencurix, Janssen, Merck Sharp & Dohme, Ono Pharma, Panagene, Pfizer, Roche, and Yuhan. I-JO received honoraria from Amgen, AstraZeneca, Eli Lilly, Janssen, Menarini, Merck Sharp & Dohme, Novartis, Pfizer, and Yuhan. All other authors have no competing interests.