Neoadjuvant Nivolumab Therapy for Esophageal Squamous Cell Carcinoma: A Single-Arm, Phase II Study
Article information
Abstract
Purpose
Programmed death-1/programmed death-ligand 1 (PD-L1) inhibitors have shown efficacy in metastatic esophageal squamous cell carcinoma (ESCC) therapy. However, data is still limited regarding neoadjuvant immunotherapy for operable ESCC.
Materials and Methods
Patients with clinical stage T2 or T3 and N0 ESCC received three cycles of nivolumab therapy every two weeks before surgical resection. The primary endpoint is major pathologic responses (MPR) rate (≤ 10% of residual viable tumor [RVT]).
Results
Total 20 patients completed the planned nivolumab therapy. Among them, 17 patients underwent surgery as protocol, showing MPR in two patients (MPR rate, 11.8%), including one pathologic complete response, on conventional pathologic response evaluation. Pathologic response was re-evaluated using the immune-related pathologic response criteria based on immune-related RVT (irRVT). Three patients were classified as immunologic major pathologic response (iMPR; ≤ 10% irRVT, iMPR rate: 17.6%), five as pathologic partial response (> 10% and < 90% irRVT), and nine as pathologic nonresponse (≥ 90% irRVT). The combined positive score (CPS) for PD-L1 in the baseline samples was predictable for iMPR, with the probability as 37.5% in CPS ≥ 10 (3/8) and 0% in CPS < 10 (0/9).
Conclusion
Although the efficacy of neoadjuvant nivolumab therapy was modest in unselected ESCC patients, further researches on neoadjuvant immunotherapy are necessary in patients with PD-L1 expressed ESCC.
Introduction
Esophageal cancer is the sixth leading cause of cancer-related mortality worldwide [1]. Esophageal squamous cell carcinoma (ESCC) is the predominant subtype of esophageal cancer in the Asian population [1]. A large proportion of ESCC cases are diagnosed at a locally advanced stage, treatment of which has been attempted using various strategies, including multimodality therapy [2-4].
In patients with esophageal or gastroesophageal junction cancer (clinical stage T1N1 or T2-3N0-1), CROSS trial showed that neoadjuvant chemoradiation therapy performed before surgery significantly increased overall survival (median, 49 vs. 24 months; p=0.003) compared to surgery alone [5]. However, another phase III trial (FFCD 9901), which enrolled patients with esophageal cancer (clinical stage T1-3N0 or T1-2N1) at an earlier stage than the CROSS trial, reported no significant difference in complete (R0) resection rate or overall survival between the neoadjuvant chemoradiation followed by surgery versus surgery alone groups [6]. Furthermore, neoadjuvant chemoradiotherapy was more harmful to patients with clinical stage N0 disease (T1-3N0) in terms of overall survival (multivariate hazard ratio, 1.49; 95% confidence interval, 1.00 to 2.23; p=0.05). In addition, neoadjuvant chemoradiotherapy was significantly associated with a higher in-hospital postoperative mortality rate than surgery alone (11.1% vs. 3.4%, p=0.049). Therefore, the best therapeutic approach for locally advanced lymph node-negative ESCC is debatable [7].
Over the last decade, there have been significant advances in the treatment of ESCC using programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors. Since several PD-1/PD-L1 inhibitors have shown clinical efficacy as monotherapy for previously treated ESCC [8-10], immune checkpoint inhibitors have played an important role in the treatment of ESCC in various treatment settings, from adjuvant therapy to first-line therapy in combination with platinum-based chemotherapy [11-13].
In this study, we aimed to evaluate the clinical efficacy of neoadjuvant nivolumab monotherapy for lymph node-negative ESCC. In addition, we evaluated the predictive biomarker for the efficacy and the underlying mechanism of the pathologic response to nivolumab therapy by comparing the concentrations of CD4 and CD8 T cells in both baseline and surgical specimens. Finally, we tried to find an appropriate method to evaluate the pathologic response after neoadjuvant immunotherapy for ESCC.
Materials and Methods
1. Study design and participants
This was a single-center, single-arm, phase II study. Eligible patients were aged ≥ 19 years and had histologically confirmed esophageal squamous cell carcinoma at clinical stages T2N0 or T3N0, which was determined by chest contrast computed tomography (CT), positron emission tomography with integrated computed tomography (PET/CT), and endoscopic ultrasonography (EUS). All patients were treatment-naïve for esophageal cancer.
2. Clinical and pathologic response assessment and biomarker evaluation
For clinical response evaluation, follow-up chest CT and PET/CT were performed 1-2 weeks after the third cycle of nivolumab therapy.
Pathologic response in a surgical specimen was assessed using two methods. First, it was assessed by tumor regression grade (TRG) using the Chirieac system, which has been conventionally used after neoadjuvant chemotherapy or chemoradiotherapy for ESCC: TRG 0 (residual viable tumor [RVT] 0%), TRG 1 (1%-10%), TRG 2 (11%-50%), and TRG 3 (51%-100%) [14]. The estimated percentage of the RVT was calculated relative to the total carcinoma area, including the amount of therapy-induced tissue injury, fibrosis, and regenerative changes.
Second, pathologic response was assessed using immune-related pathologic response criteria (irPRC) proposed by Cottrell et al. [15]. Tumor regression bed is a newly applied concept in the assessment of irPRC, and it includes areas of dense tumor-infiltrating lymphocytes with macrophages and tertiary lymphoid structures (TLS) (immune activation area), cholesterol clefts (tumor cell death area), and neovascularization or proliferative fibrosis (tissue repair areas). To determine irPRC, immune-related RVT (irRVT) was calculated as the percentage of viable tumor area out of the total tumor bed area, whereby the total tumor bed was the sum of regression bed, residual tumor, and necrosis [15]. Based on irRVT, pathologic response was defined as follows: immunologic pathologic complete response (iCR, 0% irRVT), immunologic major pathologic response (iMPR, ≤ 10% irRVT), immunologic pathologic partial response (iPR, > 10% and < 90% irRVT), and pathologic nonresponse (iNR, ≥ 90% irRVT).
3. Definition of pathologic down-staging
Patients were regarded as having been down-staged if the pathologic T category on surgical specimens was observed earlier than the clinical T category in a situation where there is no evidence of new cancer-positive lymph node in surgical specimens (e.g., from the clinical stage of T2N0 to the pathologic stage of T1bN0).
4. Immunohistochemistry and quantification of biomarkers
For biomarker evaluation, paired pretreatment endoscopic biopsy and surgical tumor specimens were evaluated immunohistochemically (IHC) for PD-L1, CD4, and CD8 expression. PD-L1 expression in formalin-fixed, paraffin-embedded (FFPE) tissue was assessed using the PD-L1 IHC SP263 kit on the Dako ASL48 platform (Ventana, Tucson, AZ) according to the manufacturer’s recommendations [16]. Certified pathologists manually assessed PD-L1 status under a microscope. The combined positive score (CPS) was calculated as the total number of stained tumor cells and immune cells divided by the number of viable tumor cells multiplied by 100.
FFPE immunohistochemical staining for CD4 (clone 4B12, Leica Biosystems, Nussloch, Germany) and CD8 (clone SP57, Ventana) was performed using the automated staining systems BOND-MAX (Leica Microsystems, Vista, CA) and Ventana BenchMark XT, respectively, with the OptiView DAB IHC Detection Kit (Ventana). The IHC slides were scanned at 20× using a digital slide scanner Pannoramic 1000 (3DHistech, Budapest, Hungary), and images with an area of 1 mm² were captured using the image viewing system INFINITT DPS (INFINITT Healthcare, Seoul, Korea) [17]. QuPath, an open-source software, was used to evaluate the number and percentage of CD4- and CD8-positive cells in four representative areas (two stromal areas, including the invasive front of tumor cells, and two intratumoral areas) of the stained slides. After observing optimal color deconvolution, the mean optical density of DAB-positive cells was determined [18].
5. Procedures
The patients received three doses of nivolumab (at a fixed dose of 240 mg) every 2 weeks. Surgery was planned approximately 2-4 weeks after the last dose of nivolumab.
After surgical resection of esophageal cancer, adjuvant therapy was performed as per the following study protocol: two cycles of adjuvant nivolumab were recommended if clinical benefit was achieved with neoadjuvant nivolumab therapy, wherein clinical benefit was defined as being classified as (1) having ≤ 10% RVT in surgical specimens, (2) exhibiting pathologic down-staging, or (3) showing dramatic response on chest CT or PET/CT. If none of the above was achieved, patients were recommended to receive 2-4 cycles of 5-fluorouracil/cisplatin.
Patients were monitored for adverse events according to the National Cancer Institute Common Terminology Criteria for Adverse Events (ver. 4.03).
6. Outcomes and statistical consideration
The primary endpoint of this study was the proportion of patients with major pathologic response (MPR, ≤ 10% RVT), which was compatible with TRG 0 or 1 by conventional pathologic evaluation, among the evaluable study population. The MPR rate was used to calculate sample size. We expected that neoadjuvant nivolumab therapy would lead to a 40% MPR rate. If the MPR rate was ≤ 15 %, the study treatment was considered not worthy of further investigation. To calculate the number of subjects, we used a one-sample chi-square test. Given a one-sided alpha of 0.10, power of 0.90, and drop-out rates of 5%, we calculated that enrollment of 20 patients was needed.
Secondary endpoints were safety, objective response rate on chest CT, and metabolic response rate on PET/CT. The clinical responses on chest CT and PET/CT were assessed according to Response Evaluation Criteria in Solid Tumor (RECIST) and European Organization for Research and Treatment of Cancer (EORTC), respectively [19,20]. In addition, PET/CT response was assessed based on the changes in proportions of the maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) between the PET/CT images obtained before and after nivolumab therapy. The changes in proportions are defined as follows: (value of the second test–value of the first test)/the value of the first test×100.
Results
1. Baseline characteristics
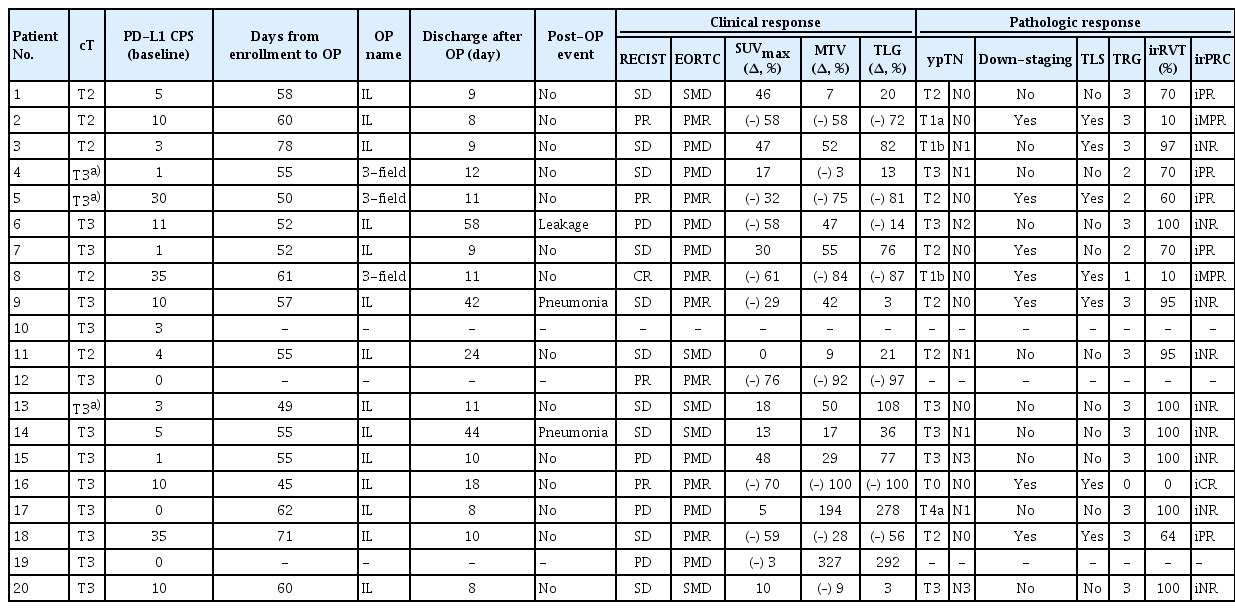
Between August 2019 and August 2021, 20 patients with esophageal squamous cell carcinoma were enrolled to receive nivolumab therapy. Table 1 summarizes the clinical characteristics of the study population, while Table 2 shows the treatment and response outcomes of all 20 patients. Five patients had T2N0 and 15 had T3N0 cancer, all of which were categorized using chest CT and PET/CT. EUS was also performed in all patients excluding three: one refusal and two technical problems due to obstructive esophageal mass (Table 2). Among the 20 patients, 17 (85.0%) and eight (40.0%) had tumors with PD-L1 CPS of ≥ 1 and ≥ 10, respectively (Table 1).
2. Treatment compliance and determination of the evaluation set
All 20 patients completed the planned three cycles of neoadjuvant nivolumab therapy and were included in the safety evaluation set (Fig. 1). Pathologic response and biomarker analysis is conducted in 17 patients, per-protocol population, who receive the surgery.
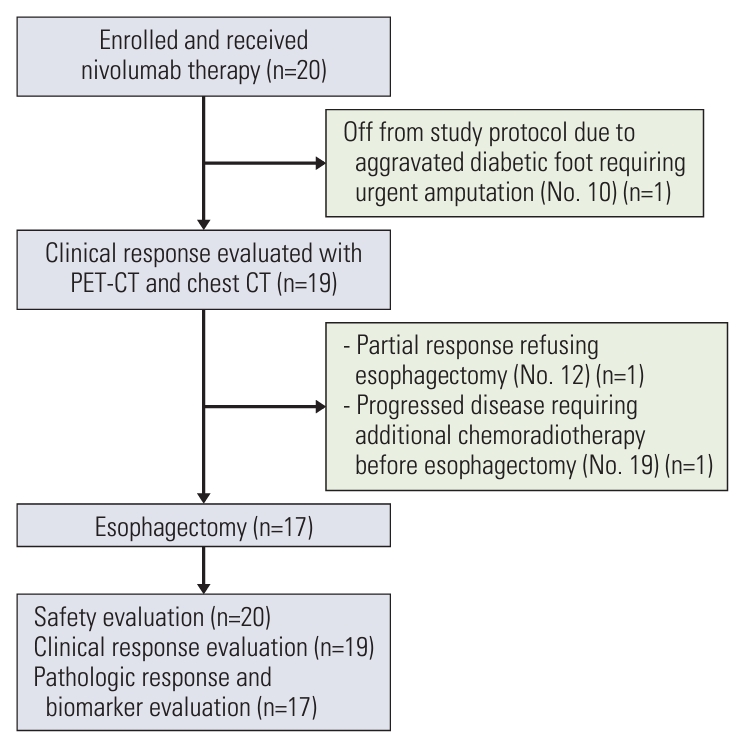
CONSORT flow diagram of the study population. CT, computed tomography; PET/CT, positron emission tomography with integrated computed tomography.
All patients were evaluated for clinical efficacy using chest CT and PET/CT, excluding one patient (clinical response evaluation set) (Fig. 1), namely, patient No. 10, who had a diabetic foot since enrollment, informed us one week after the third infusion of nivolumab that he could not follow the study protocol because his diabetic foot should be amputated at another hospital. As he also refused follow-up for esophageal cancer at our institute, he was excluded from any efficacy evaluation.
Among the 19 patients, patient No. 19 showed disease progression, as per PET/CT scans performed after three cycles of nivolumab therapy, which was evaluated by a surgeon to require additional chemoradiotherapy before surgery. Although he underwent surgery after chemoradiotherapy and there was no residual tumor in the surgical specimen, this patient was excluded from the pathologic response evaluation set. Another patient (No. 12) showed dramatic improvement in dysphagia symptoms, and there was no residual tumor in the follow-up PET/CT and endoscopic evaluation (Fig. 2A). However, he refused to undergo surgery because of the possibly decreased quality of life after esophagectomy and instead received definitive chemoradiotherapy; he is currently alive without evidence of disease recurrence. Therefore, the patient was also excluded from the pathologic response evaluation set.
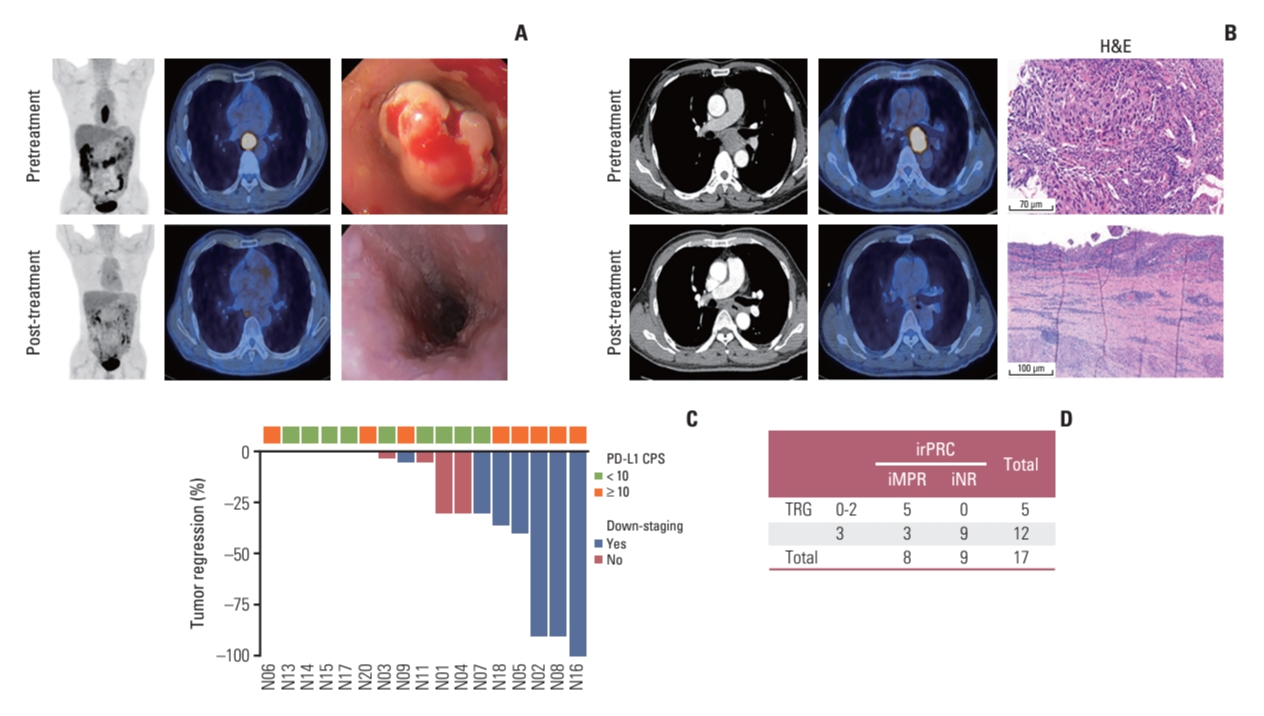
(A) PET/CT scan and endoscopic images acquired before and after treatment from patient No. 12. (B) PET/CT scan and pathologic images acquired before and after treatment from patient No. 16. (C) Waterfall plot showing irRVT based on pathologic down-staging and PD-L1 status in baseline samples. (D) Table indicating the discrepancy in positive pathologic response between conventional assessment (TRG) and irPRC. CPS, combined positive score; iMPR, immunologic major pathologic response; iNR, immunologic pathologic no response; irPRC, immune-related pathologic response criteria; irRVT, immune-related residual volume of tumor; PD-L1, programmed death-ligand 1; PET/CT, positron emission tomography with integrated computed tomography; TRG, tumor regression grade.
The remaining 17 patients proceeded to surgery in accordance with the study protocol and they were included in the pathologic response evaluation set (Fig. 1). All 17 patients underwent surgery on the scheduled date with no delay related to nivolumab therapy, and the median interval between enrollment into the study and surgery was 55 days (range, 45 to 71 days) (Table 2). The surgical procedures included the Ivor Lewis esophagectomy (n=14) and transthoracic esophagectomy with three-field lymph node dissection (n=3) (Table 2). Tumors were completely resected (R0 resection) in all the patients, with a median of 40 (range, 21 to 69) lymph nodes being surgically dissected. Three and seven patients received adjuvant nivolumab therapy and 5-fluorouracil/cisplatin chemotherapy, respectively, after surgery, according to the study protocol.
3. Safety of nivolumab therapy and perioperative complications
All cycles of nivolumab therapy were performed without interruption or skipping. Treatment-related adverse events of various grades occurred in eight of 20 patients (40.0%), and all were grade 1 or 2: skin rash (n=4, 20.0%), fatigue (n=3, 15.0%), pruritus (n=3, 15.0%), and thyroiditis (n=2, 10.0%).
Of the 17 patients who underwent surgery, one patient experienced anastomosis leakage and two patients experienced bacterial pneumonia postoperatively. However, all patients recovered with appropriate treatment, and no postoperative mortality was observed. The median period of hospitalization after surgery was 11 days (range, 8 to 58 days), and there was no event of a re-visit to the emergency room or re-hospitalization within three months after discharge from the hospital following surgery.
4. Clinical response and pathologic down-staging
Based on chest CT images, the rates of complete response, partial response, stable disease, and progressive disease were 5% (n=1), 21% (n=4), 53% (n=10), and 21% (n=4), respectively, leading to an objective response rate of 26%.
Based on post-treatment PET/CT images, the rates of partial metabolic response, stable metabolic disease, and progressive metabolic disease were 37%, 26%, and 37%, respectively. Post-treatment PET/CT images showed new lymph nodes in five patients, and the corresponding lymph nodes were confirmed to be cancer-positive in three patients and cancer-negative in two patients in surgical specimens. Cancer-positive lymph nodes in the surgical specimens of the remaining five patients had not been previously detected in PET/CT before and after treatment.
Surgical specimens from eight patients (Nos. 2, 3, 5, 7, 8, 9, 16, and 18) (Table 2) showed improved T stages compared with their clinical stages. However, patient No. 3 had an unexpected cancer-positive lymph node in the surgical specimen, leading to a total of seven cases with pathologic downstaging (Table 2).
5. Pathologic response evaluation: conventional assessment vs. irPRC
The conventional pathologic evaluation showed one TRG 0, one TRG 1, three TRG 2, and 12 TRG 3, which suggested that two patients achieved MPR (≤ 10% RVT), leading to an MPR rate of 11.8% (2 out of 17). The images of patient No. 16, who achieved a complete pathologic response (TRG 0), are shown in Fig. 2B.
The irRVT of evaluable patients using irPRC is shown in Table 2 and Fig. 2C. Based on irPRC, three patients achieved iMPR (≤ 10% irRVT), including one iCR (iMPR rate of 17.6%, 3/17), and five and nine patients showed iPR and iNR, respectively.
We analyzed the correlation between conventional pathologic evaluation and irPRC by grouping patients according to two pathologic assessments. Patients with TRG 0-2 or iMPR/iPR were segregated into a group with a positive pathologic response, while patients showing TRG 3 or iNR were segregated into a group without a positive pathologic response (Fig. 2D). Among the 17 patients, a correlation between the two pathologic assessments was detected in 14 patients: five and nine patients were categorized into a group with and without positive pathologic responses, respectively, by both assessments. However, three patients (Nos. 1, 2, and 18 in Table 2) were classified into a group with a positive response by irPRC and into a group without a positive response by conventional assessment.
For example, the surgical specimen of patient No. 2 was assessed as having no or little regressed tumor (TRG 3) but had an irRVT of 10% (iMPR) by irPRC. In comparison with his pretreatment specimen (Fig. 3A), large fields on the surgical specimen were composed of extensive immune cells, sometimes forming TLS with patchy neovascularization (Fig. 3B). In addition, the number of CD4 or CD8 T cells increased in the surgical specimens compared with pretreatment samples after nivolumab therapy, and these cells existed in intratumoral and stromal areas (Fig. 3B). In addition, tumors invading muscle layer on EUS at initial staging work-up were found only within muscularis mucosa layer on the surgical specimen (pathologic down-staging from clinical T2 category to pathologic T1a category) (Fig. 3A and B). Likewise, total two (Nos. 2 and 18) out of three patients who were reclassified into the group with a positive pathologic response by irPRC from a group without positive response by conventional assessment experienced pathologic down-staging.
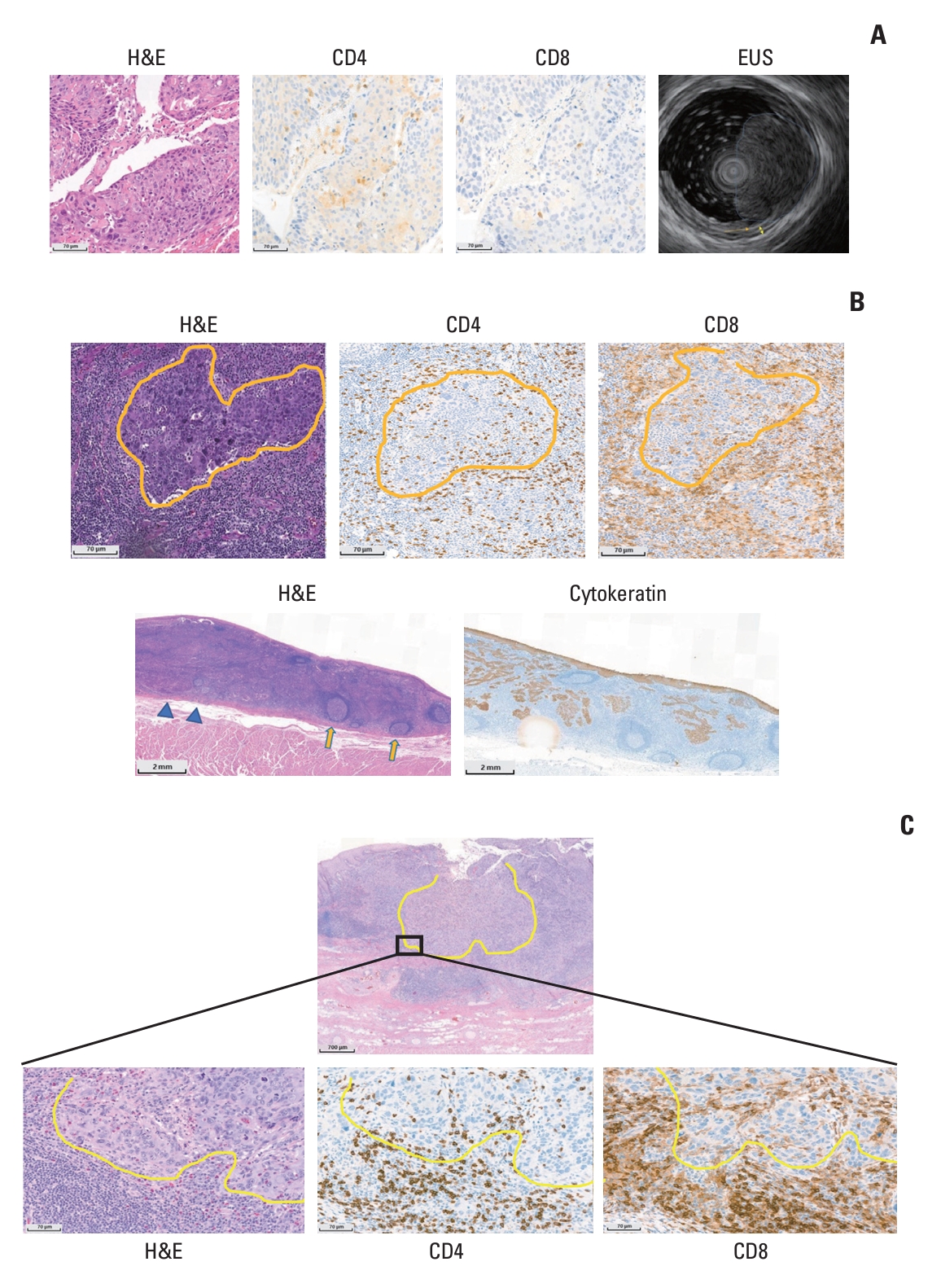
(A) Pretreatment images of patient No. 2: hematoxylin and eosin (H&E) staining shows squamous cell carcinoma with moderate differentiation. CD4 or CD8 cells are sparse in intratumoral areas or in the stroma. Endoscopic ultrasonography imaging shows that tumor invades muscle layer (marked with yellow double-arrow) and passes the submucosal layer (marked with brown arrow), suggesting clinical T2 disease. (B) Post-treatment images of patient No. 2: H&E staining shows tumor islands (yellow lines) surrounded by extensive inflammatory cells with neovascularization. CD4 and CD8 cells are present in the intratumoral and stromal areas (yellow lines indicate tumor islands). The scan view of a resected esophageal specimen shows that the tumor does not invade the muscularis propria (blue triangles indicate muscularis mucosa). There are many tertiary lymphoid structures (yellow arrows) within the tumor bed and in the surrounding tissue. Cytokeratin staining shows scattered tumor islands in the tumor bed. (C) Post-treatment images of patient No. 8: yellow lines indicate tumor margins differentiating tumor from stromal immune cells, and high-power images show that immune cells, including CD4 and CD8 T cells, infiltrate into intratumoral area and cross tumor margins from outside the tumor mass.
At the time of data analysis, a total of four patients showed postoperative esophageal cancer recurrence, all of whom had been classified into a group without a positive pathologic response (TRG 3 and iNR).
6. Predictive role of PD-L1 expression for iMPR and TLS
Out of seven patients with baseline tumors expressing PD-L1 CPS ≥ 10, three achieved iMPR (iMPR rate, 37.5%, 3/8), while there was no iMPR case in patients with PD-L1 CPS < 10.
In addition, among seven patients with baseline tumor expressing PD-L1 CPS ≥ 10, TLS were detected in surgical specimens in five patients, while TLS were found in only two patients among 10 patients with PD-L1 CPS < 10.
7. Relationship between CD4 or CD8 T cells and irRVT
As in patient No. 2 with iMPR, a dramatic increase in CD4 or CD8 T cells after nivolumab therapy was observed in patient No. 8 with iMPR. Hematoxylin and eosin staining of the surgical specimen from patient No. 8 showed a shrunken tumor island surrounded by extensive immune cells, and immunohistochemical staining slides showed CD4 and CD8 T cells infiltrating into the intratumoral area from outside the tumor (Fig. 3C).
To evaluate the relationship between baseline T cell counts and changes in T cell counts after nivolumab therapy with irRVT, patients were grouped into three response groups according to irRVT (good responders ≤ 10% irRVT; moderate responders > 10% and < 90% irRVT; poor responders ≥ 90% irRVT). Subsequently, the average count (%) of CD4 or CD8 T cells and their sum in the baseline samples were compared among the three groups. The number of baseline CD8 T cells was highest in good responders, while it was lowest in poor responders (p=0.037 by ANOVA test). There was also a similar relationship between baseline CD4 cells or the sum of CD4 and CD8 T cells in the three response groups (Fig. 4A-C). Comparative analysis of T cells in surgical samples and baseline samples showed an increase in CD4 or CD8 T cells and their sum in all three response groups after nivolumab therapy. Similar to the comparison of baseline T cell counts, however, the increase in CD4 or CD8 T cells and their sum was highest in good responders and lowest in poor responders (Fig. 4D-F).
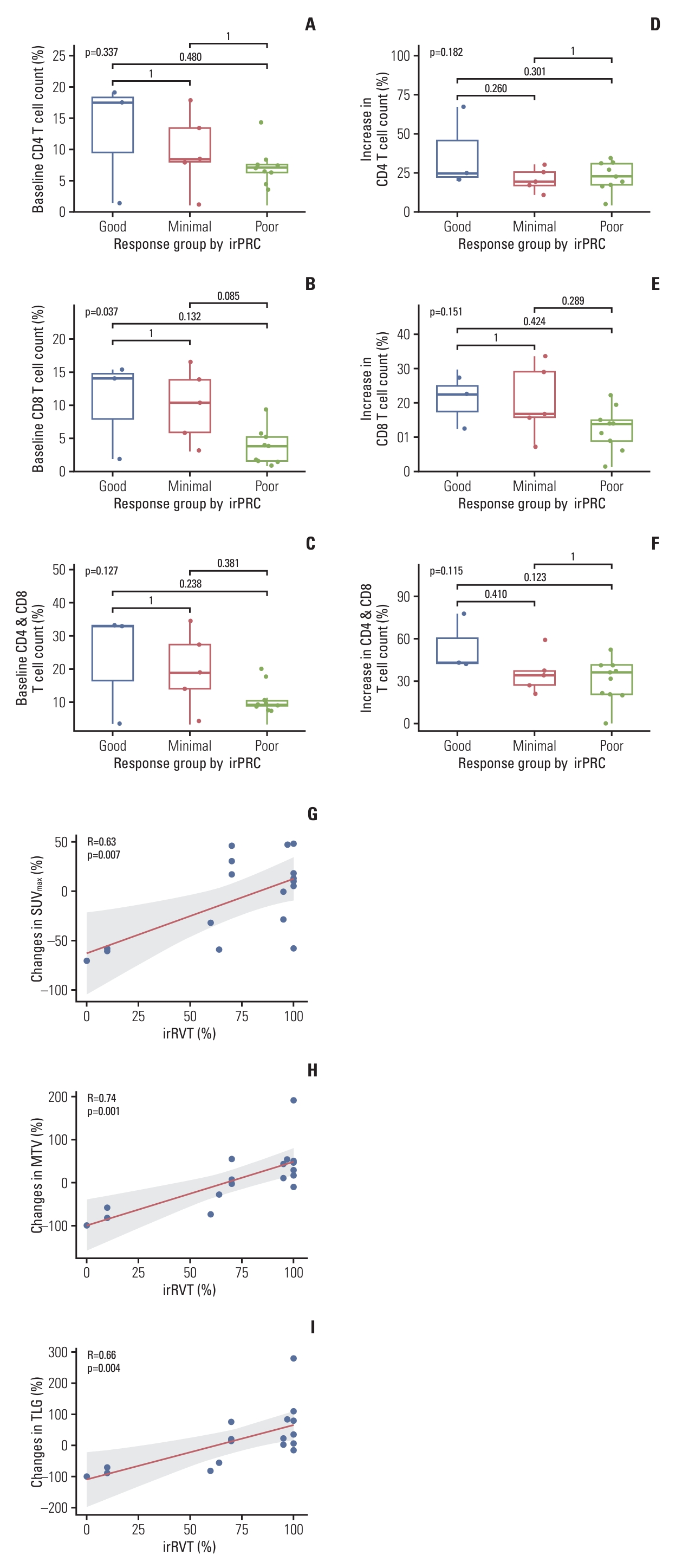
Comparison of the counts of CD4 (A), CD8 (B), and sum of CD4 and CD8 cells (C) in the baseline samples among the three pathologic response groups assessed by irPRC. Comparison of the changes in average count (%) of CD4 (D) and CD8 (E) cells and their sum (F), after nivolumab therapy, among the three pathologic response groups assessed using irPRC. Correlation of irRVT with changes in SUVmax (r=0.63) (G), MTV (r=0.74) (H), and TLG (r=0.66) (I). irPRC, immune-related pathologic response criteria; irRVT, immune-related residual volume of tumor; MTV, metabolic tumor volume; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis.
8. Relationship between PET/CT parameters and irRVT
The correlation between PET/CT parameters and irRVT was evaluated. There were moderate positive correlations between the irRVT and SUVmax (Pearson’s r=0.60), MTV (r=0.54), and TLG (r=0.49), in terms of post-treatment PET/CT parameters (S1 Fig.). Furthermore, the correlation became remarkable when irRVT was compared with changes in the proportion of the PET/CT parameter values after nivolumab therapy: there was a high positive correlation of irRVT with changes in MTV (r=0.74), followed by changes in TLG (r=0.66) and changes in SUVmax (r=0.63), in the order of decreasing correlation (Fig. 4G-I).
Discussion
Our study failed to meet its primary endpoint, showing MPR rate of 12% in the conventional assessment and 18% in the irPRC assessment. However, the current study showed that MPR rate was increased up to 43% in patients with tumors having PD-L1 CPS ≥ 10. The outstanding efficacy of neoadjuvant nivolumab in patients with PD-L1 highly expressing tumors is compatible with the results reported in previous clinical trials on PD-1/PD-L1 inhibitors for different stages of esophageal cancer [9,12,21,22].
In this study, we tried to evaluate the appropriate pathologic response method after neoadjuvant PD-1/PD-L1 inhibitors. In the pathologic evaluation after neoadjuvant therapy, MPR has been used as an independent surrogate factor for survival outcomes in patients with various cancer types and as a primary endpoint in neoadjuvant trials [23-26]. However, as PD-1/PD-L1 inhibitors have been used as neoadjuvant therapy, new pathologic evaluation has become necessary since a distinctly different pattern of pathologic response has been observed after immunotherapy compared with chemotherapy [15,27]. Based on a pivotal study of neoadjuvant nivolumab therapy for non–small cell lung cancer [26], irPRC was proposed as a novel method for evaluation of the efficacy of neoadjuvant immunotherapy [15]. Investigators showed higher inter-pathologist reproducibility of RVT scores with irPRC than conventional assessment [15] but were unable to assess its relationship with pathologic outcomes. In the current study, we showed that irPRC was more correlated with pathologic down-staging than the conventional evaluation. In one patient (No. 2) who was reclassified by irPRC into the group with a positive pathologic response from a group without a positive response (conventional assessment), pathology images showed “immune-activated” status, defined as the presence of infiltrating immune cells in both tumoral and stromal areas [15], indicating achievement of pathologic down-staging.
In addition, TLS, which is not routinely considered in the conventional assessment, was an important factor for assessment by irPRC. In our study, TLS were found in surgical specimens of seven patients, of whom six achieved pathologic down-staging, suggesting TLS may be related with good prognosis (Table 2). TLS are regarded as important sites for the initiation and/or maintenance of local and systemic T- and B-cell antitumor responses, and the presence of TLS was reported to be related to favorable survival outcomes [28].
A threshold of 10% RVT has been used to define clinically meaningful MPR by conventional pathologic assessment. However, it is unknown whether this threshold is clinically relevant for predicting clinical outcomes in the pathologic evaluation using irPRC, considering that immunotherapy has a different antitumoral mechanism and more durable effects than chemotherapy or radiotherapy. Our biomarker study showed that a difference in baseline T cell counts and their increase during nivolumab therapy occurred in sequential order in the good (0%-10% irRVT), moderate (> 10% and < 90% irRVT), and poor responders (≥ 90% irRVT). This suggests that the active antitumor effect of T lymphocytes exists even in moderate responders, and there is a possibility that the antitumor effect of systemically activated immune cells could prevail after surgery. Further research is required to determine the clinical relevance of moderately responding tumors to neoadjuvant immunotherapy.
Chest CT and PET/CT were used to evaluate the clinical response to nivolumab therapy. Although CT imaging is commonly used in clinical evaluation for response of esophageal cancer to treatment, primary esophageal tumors (e.g., digestive organ tumors) are debatable as measurable lesions because they are sometimes not large or clear enough for appropriate measurement [29]. In this context, PET/CT appears more useful in predicting the pathologic response in our study population. In particular, changes in PET/CT parameters correlated well with irRVT. In our current study, changes in MTV were most strongly correlated with irRVT (coefficient r=0.72), although SUVmax has been the most frequently used parameter in clinical practice because its measurement is simple and observer-independent. Our data are supported by the fact that SUVmax does not represent the whole tumor metabolic burden because it is analyzed from only one voxel [30].
Although PET/CT was found to be valuable in predicting the clinical or pathologic response in our study, we found that it had still a limitation in the detection of metastatic lymph nodes. There were eight patients with cancer-positive lymph nodes in their surgical specimens. However, only three patients were suspected to have cancer-positive nodes on post-treatment PET/CT, while the remaining five patients were not suspected based on all clinical evaluations performed before and after nivolumab therapy, suggesting that it is highly probable that the lymph node already existed before administration of nivolumab therapy. The inaccurate clinical staging for lymph nodes (N category) is well known; one retrospective study showed that among 482 patients clinically staged as T2N0, 184 (38%) unexpectedly had cancer-positive lymph nodes in surgical specimens [31]. As another limitation, we were not able to complete the EUS in three patients at the screening, which limited the exact evaluation of clinical staging.
To the best of our knowledge, this is the first report on neoadjuvant PD-1/PD-L1 inhibitors for esophageal cancer. Although there were no cases of failed complete resection after nivolumab therapy in our study, the clinical or pathologic response fell short of our expectation, due to which we cannot suggest further research with a similar study design. However, high probability of tumor regression after nivolumab therapy in patients with tumors having high PD-L1 expression suggests that further researches on neoadjuvant PD-1/PD-L1 inhibitors may be warranted in a selected population. Furthermore, neoadjuvant therapy of PD-1/PD-L1 inhibitors combined with chemotherapy are currently investigated for ESCC in many clinical trials [32], and this method appears promising based on previously reported data from several single-arm studies [25,33].
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study was approved by the ethics committee at Samsung Medical Center (IRB no. 2019-01-147). The study was conducted according to the recommendations of Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent to participate in the study.
Author Contributions
Conceived and designed the analysis: Park S, Lee Y, Lee J, Choi YL, Shim YM, Sun JM.
Collected the data: Park S, Lee Y, Lee J, Min YW, Kim HK, Chi JY, Jung HA, Choi YS, Choi YL, Shim YM, Sun JM.
Contributed data or analysis tools: Park S, Lee Y, Lee J, Choi YL, Shim YM, Sun JM.
Performed the analysis: Park S, Lee Y, Lee J, Choi YL, Shim YM, Sun JM.
Wrote the paper: Park S, Lee Y, Lee J, Min YW, Kim HK, Choi JY, Jung HA, Choi YS, Choi YL, Shim YM, Sun JM.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.