Intensity-Modulated Radiation Therapy for Uterine Cervical Cancer to Reduce Toxicity and Enhance Efficacy – an Option or a Must?: A Narrative Review
Article information
Abstract
Radiotherapy (RT) is a fundamental modality in treatment of cervical cancer. With advancement of technology, conventional RT used for external beam radiotherapy (EBRT) for over half a century has been rapidly replaced with intensity-modulated radiation therapy (IMRT) especially during the last decade. This newer technique is able to differentiate the intensity of radiation within the same field, thus reduces the inevitable exposure of radiation to normal organs and enables better dose delivery to tumors. Recently, the American Society for Radiation Oncology has released a guideline for RT in cervical cancer. Although a section of the guideline recommends IMRT for the purpose of toxicity reduction, a thorough review of the literature is necessary to understand the current status of IMRT in cervical cancer. This narrative review updates the recent high-level evidences regarding the efficacy and toxicity of IMRT and provides a better understanding of the most innovative techniques currently available for EBRT enabled by IMRT.
Introduction
Uterine cervical cancer is globally the second most common malignancy with the third highest cancer mortality among women worldwide [1]. Radiotherapy (RT) is an essential component of cervical cancer treatment. For locoregionally advanced disease without distant metastasis, definitive external beam radiotherapy (EBRT) with concurrent platinum-based chemotherapy followed by brachytherapy is the standard of care. In early disease, pelvic RT is considered in an adjuvant setting for patients with higher risk of relapse such as large tumor size, significant depth of invasion, lymphovascular, parametrial, nodal and/or resection margin involvement in surgical pathology [2].
The first implementation of RT for cervical cancer dates back to the dawn of 20th century, when Margaret Cleaves used intracavitary brachytherapy to treat locally advanced cervical squamous cell carcinoma in 1903 [3]. EBRT to the whole pelvis for loco-regional control became available in the mid-20th century, when high energy linear accelerators in the current form have been developed [4]. Pelvic radiation treatments in the early days were given by the 2-dimensional (2D) technique, using bony landmarks on X-ray films to determine the radiation fields. With the advent of computed tomography (CT), EBRT began to be delivered using the 3-dimensional (3D) technique (Fig. 1). Simulation CT is obtained to delineate the targets and organs at risk (OARs) on each slice of axial image. RT is planned by calculating dose on the 3D reconstructed targets for optimal delivery of radiation and sparing of OARs from unnecessary exposure. Registration of simulation CT with the portal image acquired by the treatment machine before initiation of RT allows higher accuracy compared to the conventional 2D technique. The 3D-conformal radiotherapy (CRT) has been established as the standard of EBRT by the end of 20th century [5].

Conceptual image of 2-dimensional (2D), 3-dimensional conformal (3D), and intensity-modulated radiotherapy (IMRT) showing the differences in high dose irradiated area (red solid line) and radiation intensities (black arrows). CT, computed tomography; MRI, magnetic resonance imaging; OAR, organs at risk; PET, positron emission tomography.
Although the 3D-technique enables multi-directional delivery of beams and therefore improves the conformation of radiation with target volumes, the intensity of radiation within the same field is still uniform. In 1982, the intensity modulated radiation therapy (IMRT) was first developed by Brahme, a Swedish medical physicist [6]. IMRT can deliver different intensities of radiation within the same field by modulating the shape of aperture through which radiation beamlets are projected. This technique further enhances the beam conformality and has widened the therapeutic window of RT by achieving greater dose to target volumes while reducing the exposure of OARs [7].
The potentials of IMRT has been initially demonstrated in the dosimetric studies reporting superior target coverage and better sparing of normal organs. Numerous retrospective comparisons of IMRT with conventional techniques accumulated evidence for initiation of several prospective trials. This modern technique has ignited further cutting-edge innovations to maximize treatment efficacy. The dose escalation optimized by IMRT dosimetry launched the dose escalation studies on nodal as well as primary diseases. IMRT also enables the delivery of higher dose per fraction, which gave rise to the hypofractionation IMRT trials. A more recent technique is the adaptive RT, which reduces the irradiated volume in line with the tumor volume shrinkage during the course of radiation treatment. Because IMRT allows the delivery of highly conformal beam with high enough dose to achieve durable local control, positron emission tomography (PET)–adoptive IMRT has also become more actively used in the oligometastatic setting. Attributable to the above opportunities opened by IMRT, adoption of IMRT for cervical cancer has globally expanded in the recent years [8]. Naturally, the question of whether IMRT should become a new standard or whether it remains to be just an option emerges. This review is aimed to investigate the effects of IMRT on tumor control and toxicity profiles in the literature. In addition to the prospective trials comparing IMRT with conventional RT (2D or 3D RT), newer techniques using IMRT are reviewed. The potential benefits, unsolved issues, and future directions for IMRT are also discussed. This review provides a summary of the role of IMRT in treatment of uterine cervical cancer based on the most recent evidences.
Initiative Studies
1. Dosimetric studies
With growing prevalence of IMRT, numerous studies sought to demonstrate the theoretical advantages of IMRT over conventional RT particularly in regards with dosimetry, which focuses on calculating the absorbed dose and optimizing the dose delivery in RT planning. One of the early reports demonstrates significantly lesser volume of small intestine, rectum, and bladder irradiated in cervical cancer patients undergoing pelvic and para-aortic nodal irradiation of 45 Gy [9]. IMRT demonstrates satisfactory coverage of the target volume over 98% of the prescribed dose while significantly reducing gastrointestinal (GI) toxicity up to 30% compared to conventional whole pelvic RT [7]. The results are similar even when the prescription dose is intensified up to 60 Gy for bulky lymph node (LN) [10]. Significantly lower dose to the rectum, small intestine, and bladder translates to not only significant reduction of acute proctitis, enteritis, and cystitis, but also myelosuppression and dermatitis. Chronic GI and genitourinary (GU) toxicities are also significantly reduced. A normal tissue complication probability analysis in patients treated with IMRT reports that per 100 mL of bowel receiving 45 Gy spared, acute grade 2 GI toxicity is reduced by 50% [11]. With regards to the extended-field RT, volume of duodenum receiving 55 Gy below 15 mL reduces the risk of grade ≥ 2 duodenal toxicities while still allowing dose intensification to the involved LNs up to 60-66 Gy [12]. Significant reduction in pelvic bone marrow (BM) irradiation results in significantly less incidence of anemia and thrombocytopenia in cervical cancer patients undergoing pelvic RT alone, which demonstrates the isolated impact of radiation on BM without additional influences of chemotherapy [13].
2. Retrospective studies
In line with the dosimetric studies above, retrospective comparisons of IMRT to conventional RT in the adjuvant setting demonstrate significant reduction of both acute and chronic toxicities. Reducing the OAR dose using IMRT translates to significantly lower incidence of acute hematologic, upper & lower GI, and GU toxicities [13-15]. In case of late toxicities, significant improvement is found in GU and GI toxicities in IMRT. A retrospective study specifically focusing on bowel obstruction reports that IMRT reduces the 5-year bowel obstruction rate by 10-fold compared to 3D CRT (0.9% vs. 9.3%; p=0.006) [14]. However, no difference in progression-free survival (PFS) or overall survival (OS) between IMRT and 3D CRT is demonstrated in the adjuvant setting.
On the other hand, retrospective studies in the definitive setting not only report superior toxicity profiles but also improved PFS, cause-specific survival (CSS), and OS in the IMRT arm. In a study reporting superior PFS (65% vs. 57%, p=0.04), CSS (69% vs. 62%, p=0.01), and OS (61% vs. 57%, p=0.04) at 5 years for IMRT arm, disease control is most improved in patients with involved LNs [16]. Even with better efficacy, IMRT is still found to have significantly less grade ≥ 3 GI and GU toxicities. The prescribed dose of 50 Gy in the study did not differ between the arms. When escalated dose is prescribed to the involved LNs (61.5 Gy vs. 50.8 Gy, p=0.046), IMRT shows further significant improvement in 5-year PFS (64.9% vs. 44.3%, p=0.031) [10]. Significantly less acute hematologic toxicities encompassing leukopenia, neutropenia, anemia, and thrombocytopenia are reported in IMRT compared to 3D CRT [17].
Landmark Studies and Current Guidelines
1. Definitive setting
The benefits in survival and toxicity profiles previously observed in the retrospective studies has been demonstrated in a large prospective cohort study (n=452) which finds the IMRT arm to have significantly better CSS and OS compared to conventional RT [18]. The rate of chronic grade ≥ 3 GI and GU toxicities is 6 times less in IMRT (8 vs. 54, p=0.035). Three randomized prospective trials in the definitive setting also report fewer acute and chronic toxicities in favor of IMRT compared to conventional RT. However, none of these trials has found difference in disease-free survival (DFS) or OS between IMRT and conventional RT. A phase III trial by Gandhi et al. [19] report that patients treated with IMRT significantly experience fewer grade ≥ 2 (31.8% vs. 63.6%, p=0.034) and grade ≥ 3 acute GI toxicities (4.5% vs. 27.3%, p=0.047). IMRT is also associated with less chronic GI toxicities (IMRT 13.6% vs. conventional 50%, p=0.011). The phase III trial by Yu et al. [20] demonstrates that the incidence of grade ≥ 3 acute enteritis is lower (5.6% vs. 30.6%) in IMRT. Naik et al. [21] report significantly lower rate of acute grade ≥ 2 (20% vs. 45%, p=0.003) and grade ≥ 3 (5% vs. 20%, p=0.004) GI toxicities and lower rate of acute grade ≥ 3 GU toxicities (5% vs. 15%, p=0.004) in IMRT. A meta-analysis of IMRT in the definitive setting corroborates the above results by concluding that IMRT demonstrates equivalent efficacy with respect to DFS and OS at 3 years while significantly reducing acute GI and GU toxicities in addition to chronic GU toxicity [22].
2. Adjuvant setting
Consistent with the retrospective studies, prospective studies on IMRT in the adjuvant setting demonstrate satisfactory efficacy and safety [23,24]. A prospective trial reports OS rate of 100% and PFS of 89% at 3 years [25]. Chronic grade ≥ 3 GI and GU toxicity rates are each 4%. Another prospective study investigated the relationship between bowel irradiation and late grade ≥ 3 toxicity [26]. This group suggests to keep the volume irradiated by 15 Gy < 275 mL for small bowel and < 250 mL for large bowel in order to reduce the late grade ≥ 3 toxicity rate below 5%.
Key randomized trials comparing IMRT to conventional RT in the adjuvant setting are the TIME-C (NRG Oncology – RTOG 1203) trial and the PARCER (Postoperative Adjuvant Radiation in CERvical cancer) trial (Table 1). The TIME-C trial assessed patient-reported acute toxicity and quality of life during treatment [27]. Both the acute GI (p=0.048) and GU toxicities (p=0.03) are significantly less in the IMRT group. At the end of RT, fewer patients in the IMRT group are found to suffer from diarrhea (33.7% vs. 51.9%, p=0.01) and require frequent antidiarrheal agent (7.8% vs. 20.4%, p=0.04). However, most of the patients included in the TIME-C trial were endometrial cancer and cervical cancer constituted less than 20% of the patient population, owing to the aim of the study for assessment of acute treatment-related toxicity only, of which why further analysis of treatment outcome is unfeasible. On the other hand, the PARCER trial was conducted in a pure cohort of cervical cancer patients [28]. It reports significantly lower rate of any grade ≥ 2 late toxicity (28.1% vs. 48.9%, p < 0.001) and significantly fewer grade ≥ 2 late GI toxicity (21.1% vs. 42.4%, p < 0.001) in IMRT. The PARCER trial also reports oncologic outcome and there is no significant difference in 3-year pelvic relapse-free survival (81.8% vs. 84%, p=0.55) and DFS (76.9% vs. 81.2%, p=0.089) between IMRT and conventional RT.
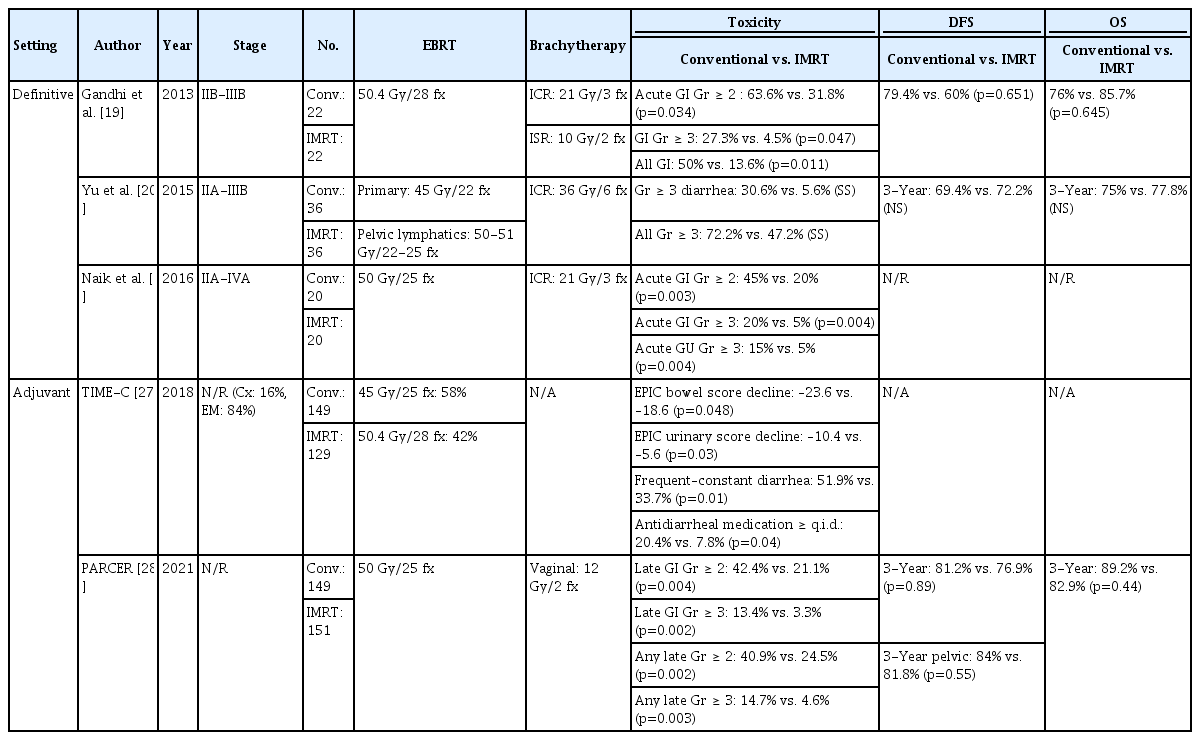
Key prospective phase III randomized controlled trials comparing intensity-modulated radiation therapy with conventional radiotherapy
One notable issue regarding the trials in adjuvant setting is the method of toxicity assessment. The TIME-C trial emphasizes the importance of patient-reported outcome for toxicity evaluation. The insignificant difference in late toxicity between IMRT and conventional RT according to physician-reported outcome becomes significant when the analysis is performed using patient-reported outcome [29]. A recent post-hoc analysis of the PARCER trial also demonstrates superior discriminative ability of adverse event reporting by patients compared to common toxicity evaluation tool used by physicians such as the Common Terminology Criteria for Adverse Events (CTCAE) [30].
3. BM sparing
The adoption of technological advance in RT for cervical cancer has generated a whole new field of BM sparing RT. In addition to the benefits of IMRT in reducing GI and GU toxicities, several studies explored the dosimetric advantage of IMRT in reducing hematologic toxicities. An early study reports that the volume of pelvic BM irradiated is significantly less in IMRT [31]. The BM volume irradiated by 10 Gy (V10) is significantly related to grade ≥ 2 leukopenia [32]. Another dosimetric study suggests V10 < 95% and V20 < 76% for reduction of grade ≥ 3 leukopenia [33]. Integration of 18F-fluorodeoxyglucose (18F-FDG)-–positron emission tomography/computed tomography (PET/CT) to define active BM subregions demonstrates that hematologic toxicities such as nadir of leukocyte, neutrophil, hemoglobin, and platelet are associated with active BM with higher activity in FDG-PET/CT [34]. Another study using FDG-PET/CT to observe longitudinal changes in active BM reports that patients have variable subacute compensatory BM responses after definitive concurrent chemoradiotherapy (CCRT) due to differing recovery in unirradiated BM subregions, which is particularly vulnerable to intensive systemic chemotherapy [35].
In a prospective randomized controlled trial comparing BM-sparing IMRT to IMRT without BM constraints in the definitive setting, BM-sparing IMRT significantly lowers grade ≥ 2 hematologic toxicities by 20% [36]. RTOG 0418 is a multi-institutional phase II trial of IMRT in the adjuvant setting to test its feasibility for cervical and endometrial cancer. In an analysis focusing on the hematologic toxicity of RTOG 0418 trial, BM V40 < 37% and median BM dose < 34 Gy are significantly associated with reduced grade ≥ 2 toxicity [37]. The INTERTECC (INTernational Evaluation of Radiotherapy Technology Effectiveness in Cervical Cancer) trial is a phase II/III trial testing the efficacy of PET/CT guidance in BM-sparing IMRT (Table 2) [38]. The phase III trial originally randomized patients to PET-guided BM-sparing IMRT versus conventional RT with PFS as the primary endpoint but was terminated early due to futility. The phase II component of the trial compared the above two arms without randomization and reports significantly reduced acute GI and hematologic toxicity for PET-guided BM-sparing IMRT, both in definitive and adjuvant setting [39]. In the final analysis including phase III patients, PET-guided BM-sparing IMRT significantly reduces acute grade ≥ 3 neutropenia compared to standard IMRT [38].
4. ASTRO (American Society for Radiation Oncology) Guideline
The capability of IMRT for reducing toxicity has been repeatedly proven in several trials. However, the absence of high-level evidence for oncologic benefit of IMRT compared to conventional RT leaves the question asked at the introduction of this review—whether IMRT remains to be just an option or not—also for the working group of ASTRO Cervical Cancer Guideline. Currently, the recommendation of IMRT for cervical cancer is limited to adjuvant setting. The ASTRO guideline ‘strongly’ recommends IMRT in women with cervical cancer with postoperative RT in order to decrease acute and chronic toxicity [40,41]. The strength of recommendation is reduced to ‘conditional’ in women with cervical cancer treated with definitive RT. Current recommendations are based on the toxicity reducing effect of IMRT with greater magnitude in the adjuvant setting compared to the definitive setting. The benefits of toxicity reduction are more apparent in the adjuvant setting, in patients who are more sensitive to radiation toxicities superimposing on the aftereffects of surgery. However, growing body of literature point towards the potential benefits of IMRT for improving tumor outcome.
Potential Benefits
1. Enhancing local control
1) Systemic therapy
Although non-bulky tumors are well controlled with the current standard of concurrent chemoradiation followed by brachytherapy, loco-regionally advanced disease still necessitates further improvement of tumor outcome. The attempts to improve durability of treatment have been tried in diverse aspects including intensifying systemic therapy as well as local therapy. The recent preliminary report of the OUTBACK trial which tested the role of adjuvant chemotherapy after completion of standard chemoradiation in locally advanced cervical cancer shows negative results [42]. Adjuvant chemotherapy has no effect on OS or PFS and instead, it is associated with approximately 20% higher rate of toxicity.
2) Brachytherapy
The failure of additional systemic therapy in improving tumor outcome draws attention to intensifying local therapy. The most effective form of consolidative therapy on the primary site is brachytherapy rather than EBRT [43]. Recent efforts for dose escalation on the primary site use the image-guided adaptive brachytherapy. Similar to EBRT, technological advances have enabled 3D application of brachytherapy with either CT or magnetic resonance imaging (MRI), allowing higher dose delivery to primary disease and lowering radiation exposure of OARs. The multicenter retrospective RetroEMBRACE study including 731 patients reports excellent local control (89%), pelvic control (84%), CSS (65%), and OS (73%) with 5%-7% severe GI and GU toxicities at 5 years [52]. These results are corroborated by the multicenter prospective EMBRACE I study showing that dose escalation to the cervical mass has significantly improved outcome up to a 5-year local control (LC) rate of 92% across stages I to IV with severe late toxicities of 3%-9% [44]. However, para-aortic nodal failure is found to be the major challenge for nodal control in RetroEMBRACE and EMBRACE I studies. In RetroEMBRACE and EMBRACE I, only 9% and 27%, respectively, were treated with IMRT and the rest were conventional RT. Consequently, EMBRACE II study is initiated to prospectively validate MRI-guided adaptive brachytherapy with the most up-to-date IMRT techniques to escalate dose to LN and de-escalate dose to normal organs [45].
3) IMRT boost with brachytherapy
Moreover, dose escalation to cervical mass by adding IMRT boost to brachytherapy also has been explored. Dose escalation by simultaneous boost to FDG-avid cervical tumor during IMRT is found to be dosimetrically feasible [53]. Dosimetric studies also demonstrate improved cervical tumor and parametrial coverage with concurrent addition of IMRT boost to brachytherapy [54-56]. A retrospective study reports LC of 88% and DFS of 76% at 5 years [57].
4) Stereotactic ablative body radiotherapy boost to brachytherapy-infeasible tumors
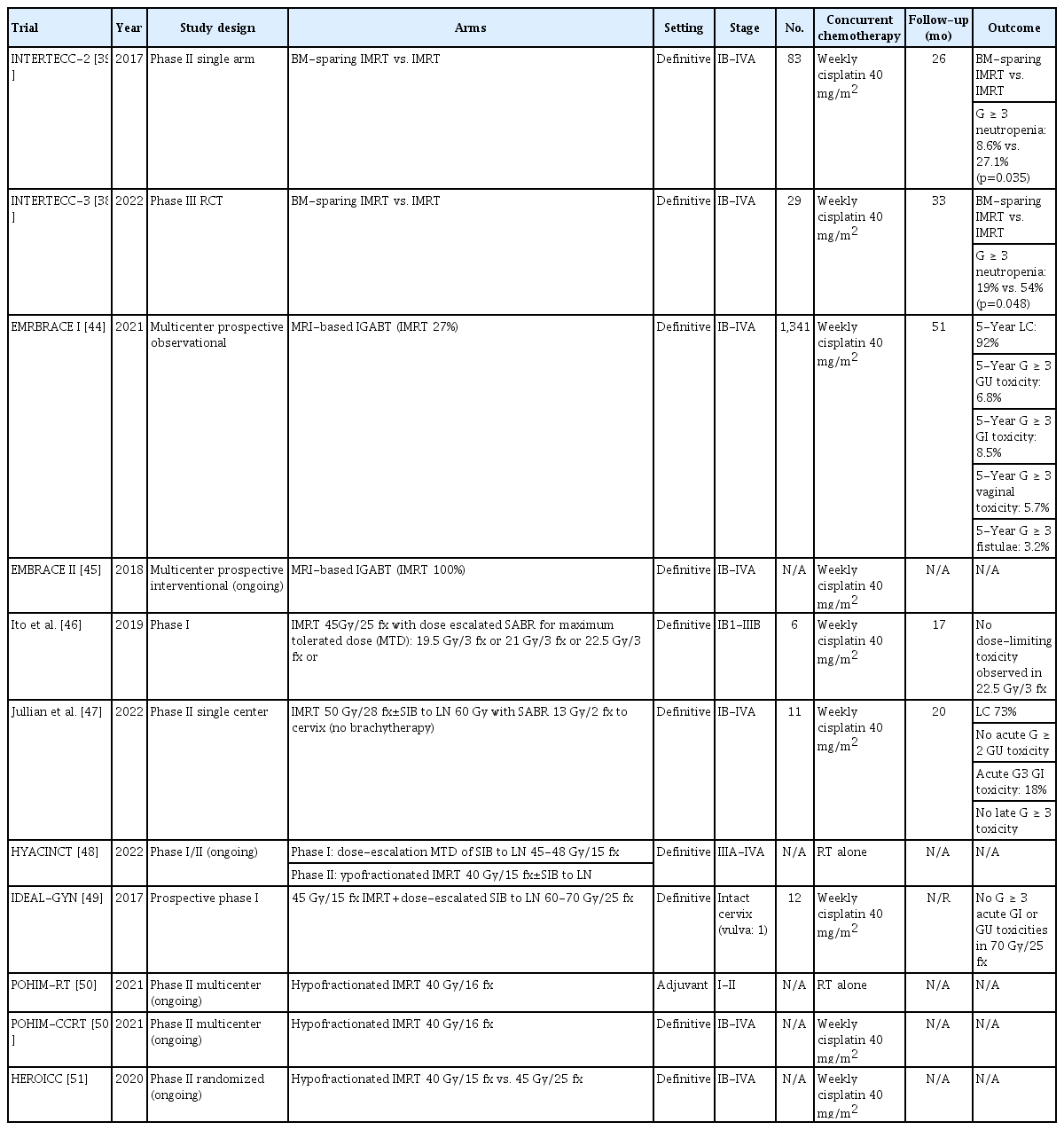
Although brachytherapy is the optimal modality for consolidative RT to the primary site, stereotactic ablative body radiotherapy (SABR) boost to the cervical mass can be an excellent alternative to brachytherapy when it is not feasible due to tumor obstruction, anatomical variation, comorbidities, and patient refusal [58]. SABR is a highly focused IMRT technique which intensifies dose concentration on tumor while effectively reducing dose to surrounding normal organs. There are few prospective trials and several retrospective studies reporting the feasibility and safety of SABR boost on cervical mass [46,59,60]. Small retrospective series report 3-year LC rate of up to 80%, modest PFS approximating 60%, and 2-year CSS rate of 90% [61]. Another multicenter retrospective study reports a 5-year PFS of 70% [60]. In a prospective single center study, LC rate is 73% without grade ≥ 3 late toxicity [47]. With lack of direct comparison between SABR and brachytherapy as the boost modality in definitive RT of locally advanced cervical cancer, National Cancer Database data shows inferior survival in patients treated with SABR boost [8]. There are concerns that widespread IMRT technique resulting in active administration of SABR instead of brachytherapy has induced detrimental effects on survival [43]. A single institutional single arm phase II study of SABR boost was closed early due to toxicity and lower-than-expected tumor outcome [59]. Despite all these limitations, SABR boost is comparable to historical results [62] and even satisfactory in patients not feasible for brachytherapy, who are likely to be senile with underlying comorbidities.
5) Hypofractionated IMRT
Another approach to improve LC using IMRT is hypofractionation. Concurrent chemotherapy improves OS by 7.5% at the expense of acceptable acute grade 3-4 toxicities [62,63]. However, there are clinical scenarios where patients are unable to receive concurrent chemotherapy such as locally advanced disease with impaired renal function, borderline cardiac function, and other comorbidities. In those cases, hypofractionated IMRT can be an effective alternative. Hypofractionated RT increases the dose per fraction to ≥ 2.5 Gy up to a total dose of 39-40 Gy in 13-16 fractions. It reduces total treatment time and is radiobiologically more effective for tumor control [64]. Retrospective studies on stage IIIB cervical cancer treated with hypofractionated conventional RT shows complete response in 70% and DFS approximating 60% at 5 years [65,66]. An ongoing I/II trial with hypofractionated IMRT uses simultaneous boost to LN in definitive RT alone without chemotherapy [48]. Another ongoing phase II trial is testing hypofractionated IMRT alone in the adjuvant setting [50]. On the other side, hypofractionated RT is also delivered with concurrent chemotherapy. In this context, hypofractionated RT is selected to further augment tumor control rather than to replace the missing effect of chemotherapy in contraindicated patients. Prospective phase I/II trial of stage IIIB cervical cancer testing hypofractionated RT given concurrently with chemotherapy reports complete response in 85% and 5-year OS of 59% [67]. There is an ongoing phase II trial for hypofractionated IMRT with concurrent chemotherapy in the adjuvant setting [50].
2. Enhancing regional control
Similar to the dose escalation trials for primary mass, several dose escalation studies for nodal disease have been conducted. Even with the most advanced local therapy, total nodal failure rates are 11%-13% in the EMBRACE-I and RetroEMBRACE studies, with higher rate of 16% in the node positive cohort [44,52]. Because extended-field RT to the para-aortic node (PAN) includes a long segment of abdominal irradiation, physicians have been reluctant to irradiate PAN for prophylactic purpose due to concerns of abdominal toxicities in the 3D era. However, IMRT has enabled effective irradiation to PAN with tolerable abdominal toxicity. Dose escalation to LN can be either delivered sequentially or by simultaneous integrated boost (SIB). Sequential boost allows adaptive delivery of radiation according to reduced nodal size during the course of RT, usually up to 55-65 Gy. SIB delivers higher daily doses of 2-2.5 Gy up to 50-60 Gy within shorter overall treatment time, which is supposed to have greater radiobiological effect at least theoretically. An example of adaptive RT to bulky LN is shown in Fig. 2. Planning studies report superior dosimetric parameters with SIB compared to conventional RT [68]. When compared to sequential IMRT boost, SIB is non-inferior [69,70]. A dose escalation study using SIB of 56.25 Gy in 25 fractions to LN reports 3-year OS, PFS, and loco-regional control rates of 67%, 60%, and 89%, respectively, in clinically node positive cervical cancer undergoing definitive CCRT [71]. Acute grade ≥ 3 GI and GU toxicities occurred in 3% and overall late GI or GU toxicity rate was 12%. In a recent trial testing newer chemotherapeutic agent in adjunction with cisplatin for definitive CCRT, SIB to LN up to 60 Gy is tolerable with complete response in > 90% [72]. PET/CT has 20%-25% false negative rate for PAN in patients with PET-positive node limited to the pelvis [73,74]. An interesting study which addresses this issue tested the value of PAN irradiation in this specific cohort of patients with pelvic nodal involvement only. They report a significantly improved PAN failure rate of 2.5%, without surgical removal of PAN [75]. However, a recent study reports reduced nodal control in cases of nodal dose below 60 Gy and large node size over 2 cm [76]. Another recent dose escalation trial tested dose intensification up to 70 Gy in 25 fractions (2.8 Gy per fraction) without any grade ≥ 3 GI or GU toxicities [49]. An ongoing multi-institutional phase II randomized trial compares two different regimens of hypofractionated IMRT given with concurrent chemotherapy to test feasibility of higher dose SIB to LN [51]. The results of these trials may bring changes to the current standards of definitive CCRT.
3. Downstaging of systemic disease
Based on the spatially sequential pattern of disease spread, intensifying local treatment in distant metastasis especially to the distant nodal regions such as the supraclavicular (SCL) have been increasingly studied in the recent years. With a curative intent, 60 Gy to the SCL metastasis along with the standard CCRT to the pelvis achieves a comparable 3-year OS rate of 49% [77]. In a study on cervical cancer patients with distant nodal metastases, CCRT shows significant survival benefit over systemic chemotherapy alone [78]. According to a more recent study, definitive RT to oligometastatic sites shows a 3-year OS rate of 65% [79]. SABR to oligometastatic cervical cancer achieves complete response in 55.4% with 2-year LC rate of 89% [80]. The 2-year OS approximated 70% in patients with complete response versus 44% in patients with suboptimal response. Excellent LC > 97% and median OS over 50 months with minimal toxicity is shown in a smaller study [79]. Major pattern of relapse is outfield with half of patients with regional or distant failure at 2 years after RT. FDG-PET/CT is essential in selection of targets for SABR as shown in ongoing randomized phase III trials testing the effect of SABR in oliogmetastatic disease which strongly recommend PET/CT for target definition [81-83]. Accordingly, precision medicine using PET-adoptive oligometastatic IMRT has the potential to further maximize treatment efficacy (Fig. 3). Owing to the radiosensitive nature of squamous cell carcinoma, tailored RT to the distant sites enabled by IMRT may contribute to improving survival, provided that potentially curable distant LN metastasis are appropriately selected (e.g., low disease burden of SCL metastasis suggesting low probability of synchronous microscopic metastasis to the next echelon of spread).
4. Prevention of pelvic insufficiency fracture
In a meta-analysis including 3,929 patients who received EBRT on the pelvis for gynecologic cancers of which majority were cervical cancer, overall pelvic insufficiency fracture (PIF) rate is 14% [84]. IMRT significantly (p=0.0299) lowers the rate of fracture (6%) and conventional RT is associated with 2.6-4.3 times more PIF. Median time to fracture is 7-19 months after completion of EBRT. The most common site of PIF is sacroiliac joint (40%), followed by sacral body (34%), pubis (13%), lumbar vertebra (7%), iliac bone (3%), acetabulum (2%), and femoral head or neck (1.5%). A direct comparison of damage to pelvic bone between IMRT and conventional RT also demonstrates significant reduction of risk in IMRT (p=0.01) [85]. When IMRT shows PIF in 4%, the rate of all pelvic bone complications in conventional RT is 17%: PIF (11%), osteonecrosis (2%), and osteomyelitis (4%). Latent osteoporosis occurred in 5% of patients treated with conventional RT. In a study on PIF of patients undergoing definitive CCRT using IMRT with SIB on the LN up to 60 Gy, 20% is shown to have a median of 2 PIFs of which half is asymptomatic [86]. Similarly, sacrum is the most common site of PIF (77%). Age is a significant risk factor for PIF and age over 50 has significantly higher rate of PIF (37% vs. 4%, p < 0.001). Dosimetrically, reduction of sacrum receiving 50% of prescription dose (D50%) from 40 Gy to 35 Gy translates to risk of PIF from 45% to 22% (p=0.04), suggesting 40 Gy as a practical cutoff for PIF. Because majority of the patients undergoing pelvic RT for cervical cancer are postmenopausal, this study draws attention to awareness for RT planning especially in patients with old age. In an observational cohort study including 28,354 patients with age ≥ 65 who underwent RT for all pelvic malignancies, IMRT significantly reduces the risk of PIF (hazard ratio, 0.85; 95% confidence interval, 0.73 to 0.99) [87]. In summary, current evidence strongly advocates IMRT for all patients requiring pelvic RT and shows that it is indispensable especially in senile patients. Because 40%-50% are asymptomatic at diagnosis of PIF, surveillance focused on bone complication is warranted [84,86]. Data regarding potential benefits of bone-strengthening medications in this specific setting are awaited.
5. Preservation of hormonal functions and fertility
With improved survival of female cancer patients at reproductive and premenopausal age [88], cancer survivorship has evolved to comprise fertility and hormonal functions. The efforts to preserve fertility and hormonal functions in gynecologic cancer patients have been attempted in various ways including minimal surgery, ovary transposition, and ovary- or uterus-protective medications. RT-wise, ovarysparing with IMRT has been shown to significantly reduce the dose irradiated to the ovaries in pelvic malignancies (Fig. 4) [89,90]. However, the goal of ovary-sparing with IMRT is to preserve hormonal function. The minimal dose acceptable for ovum is close to background dose [91], so even with ovary transposition in addition to ovary-sparing with IMRT, fertility preservation is unachievable. Transposition of an ovary to the para-colic gutter can spare the ovary from high dose irradiation, but it cannot completely obviate the radiation itself, thus allowing a certain amount of low dose irradiation to the transposed ovary. If patients wish to conceive, assisted reproductive specialist should be consulted before initiation of treatment. There also have been anecdotal reports of uterine-sparing RT with curative dose to the cervix alone which lead to successful pregnancy [89]. Uterine-sparing IMRT may be recommended as an option to the cervical cancer patients who firmly wish to conceive, although the risks of compromising cancer survival without successful preservation of fertility should be strongly acknowledged.
Unsolved Issues
1. Undefined timing, target, and dosage
With broadening implementation of IMRT as reviewed above, new problems emerge such as when the optimal timing of IMRT is, where it should be given, and how much. Advances in RT technology as well as systemic therapy are constantly re-shaping the landscape of cervical cancer treatment. Even stage IV patients with dissemination can reach the state with no evidence of disease, at least temporarily. Oligoprogression raises the question of when IMRT should be given and whether in adjunction with systemic therapy or not. Optimal dosage and regimens for IMRT to cervical cancer in various clinical settings have not been definitively established. In case of nodal oligometastasis, the target volume is undefined because whether IMRT to the isolated node is enough or addition of regional nodal irradiation is necessary is unknown. The role of newer biomarkers such as circulating tumor DNA for these kinds of clinical scenarios has been suggested [92]. This review has focused on dose escalation with IMRT to improve LC. However, salvage surgery after incomplete response to the standard definitive CCRT in locally advanced cervical cancer is another field of research under active investigation [93]. If high level evidence supporting salvage surgery is demonstrated, RT may evolve into de-escalation of dose for prevention of fibrosis, thus trading-off the benefits and the needs for IMRT.
2. Shortcomings and limitations
Another major limitation of IMRT is that it is a time-, resource-, labor-intensive, and thus expensive treatment compared to conventional RT [94]. Although current evidence shows significant reduction of toxicities and potential improvement of tumor control in IMRT, whether side effects of conventional RT are fatal is another issue. The cost-effectiveness analyses in both the definitive and adjuvant settings show questionable cost-effectiveness regarding actual clinical effectiveness by IMRT [94,95]. The ASTRO guideline is also aware of certain circumstances of shortcomings such as less provider experience and shortage of facility resources [41]. Considering the balance between resource input and clinical outcome, the current standard of conventional RT appears to be reasonably acceptable.
3. Uncertainties from organ movement
The uterine body and cervix is a highly movable organ because its position depends on the variable filling of adjacent bladder and rectum [96]. Rapid reduction of cervical tumor during the course of definitive RT is another factor affecting organ movement [97]. Cervical tumor is reported to show mean reduction of 62% after 45 Gy. A systematic review including 39 studies on cervical cancer treated with definitive IMRT reports that uterus is more mobile than cervix [98]. Inter-fractional studies show that uterine motion can range up to anterior-posterior (AP) 48 mm and superiorinferior (SI) 32 mm, compared to AP 19 mm and SI 12 mm for cervix. Bladder filling is more associated with uterine motion while rectal filling is more associated with cervical and vaginal motion. This study reports that intra-fractional cervical motion is negligible and easily covered by internal margin. However, in a study including patients with large tip-of-uterus displacement > 2.5 cm between full and empty bladder, cervical motion up to SI 5.8 mm is observed [99]. In the adjuvant setting, vaginal motion is investigated in a prospective study [100]. The median vaginal displacement is shown to be AP 2.8 mm, SI 4 mm, and medio-lateral (ML) 1.2mm. The displacement is adequately covered with internal margins of AP 10.6 mm, SI 10.3 mm, and ML 4.1 mm. In essence, a major limitation of IMRT in cervical cancer is the challenge of extrapolating how the IMRT is performed in the setting of multiple fractionated RT. It becomes a greater issue when the dose delivered by IMRT is escalated. Another limitation is that radiosensitive cervical tumors rapidly regress over the course of RT and overdose to OARs around regressed cervical tumors can obscure the benefits of IMRT conformity. Despite the development of several empirical ways to reproduce the target position such as prone position with belly board, filling the bladder with regular amount of normal saline through Foley catheter, emptying the rectum by enema, using fiducial markers, and on-line image guidance, the issue of organ movement has not been completely resolved [101-105]. These limitations draw special attention to the rapidly developing adaptive RT.
Future Perspectives
1. Adaptive RT
There are several strategies available for adaptive RT, one of which to adopt patient-based individualized margins instead of population-based margins [106]. The tip-of-uterus motion due to bladder filling ranges by AP 0-65 mm and SI 5-40 mm [107]. However, the planning target volume (PTV) remains large considering the additional margin of 5-7 mm for set-up error [103]. In order to solve this problem, offline adaptive replanning either scheduled in advance or impromptu has been studied. Weekly replanning enables IMRT with smaller margins, at the cost of increased workload required for generation of multiple plans [108]. Another approach is creating a library of plans for each patient using individualized internal margins, also known as ‘Plan-of-the-Day’ technique. The difference in PTV is median 48%, with reduction of bladder and rectal volumes included within PTV by 5%-45% and 26%-74%, respectively [109]. The number of plans required to adequately cover target volumes is median 3-5 [110]. ‘Plan-of-the-Day’ demonstrates significantly superior coverage of target volumes than standard plan with fixed margin [111]. For administration of this technique, RT therapist needs to be trained in order to select the optimal ‘Plan-of-the-Day’. This approach also requires heavy workload, twice as much compared to non-adaptive RT [112]. Another more upfront technique is online adaptive RT with daily replanning, either with MRI or CT. This strategy addresses the day-to-day anatomical variability observed in target and OAR volumes along with the daily changes in tumor volume [113,114]. Again, the implementation of these most recent techniques require additional workforce with further training, despite automation of contouring and RT plan generation are available by computational advancements [106]. Although the limitations of IMRT equally applies to adaptive RT in terms of balancing the cost-effectiveness and clinical benefits, adaptive RT is currently an active field of investigation with promising outcomes.
2. Ongoing studies
The comparison between IMRT and conventional RT appears to have become obsolete at present, although there is an ongoing phase II randomized trial specifically focusing on stage IIB cervical cancer [115]. The inclusion of IMRT as a mandatory component in the study design of majority of ongoing clinical trials on cervical cancer reflects the routine implementation of IMRT in daily clinical practice. Currently ongoing trials mostly focus on improving treatment efficacy with application of most advanced technology and novel agents. The previously mentioned EMBRACE II trial aims to maximize tumor control with cutting-edge brachytherapy in adjunction with most modern IMRT techniques [45]. Recently released results of the NRG-GY006 trial, a phase III randomized trial testing triapine plus cisplatin CCRT fails to show survival benefit [116]. Similarly, the initial results from the CALLA trial, a phase III randomized trial testing durvalumab in combination with and following cisplatin CCRT, is also negative [117]. NRG-GY017 trial is a phase II trial testing atezolizumab with cisplatin CCRT using IMRT [118]. Whether a newer agent can further enhance the disease outcome achieved with modern IMRT techniques to be revealed in the EMBRACE II trial remains to be seen. The LiLACS trial is a phase III trial comparing surgical versus clinical staging in locally advanced cervical cancer undergoing CCRT [119]. A similar Uterus-11 multicenter phase III trial reports that surgical staging leads to extended-field RT by two-fold [120]. Combining surgical approach with the newest radiation techniques may provide a breakthrough for poor prognostic subgroup, which however, requires further accumulation of evidence.
3. Future directions
As previously reviewed, there are several potential benefits of IMRT in enhancing local, regional, and even oligometastatic disease control. Currently, we are in need of a well-designed prospective trial testing the ultimate impact of IMRT with dose escalation in diverse clinical settings, with delicate control of the factors directly affecting tumor outcome such as brachytherapy, LN boost, dosage, and sequencing. Factors affecting toxicity outcome such as motion management of internal organs should also be initially planned in the trial design. With implementation of the most up-to-date technological advances such as IMRT and image-guided adaptive brachytherapy in addition to newer systemic therapies and minimally invasive surgical techniques, further prolongation of cervical cancer survival is anticipated.
Conclusion
The discussion brings us back to square one: is IMRT an option or a must? Toxicity-wise, it is a must for postoperative RT in the least. A large body of evidence support the potential impact of IMRT for improvement of local, regional, and distant control. IMRT can be indispensable in special circumstances such as where brachytherapy is infeasible. Cutting-edge adaptive IMRT maximizes treatment efficacy and minimizes treatment-related toxicities. IMRT is a must in meeting the escalating expectations of oncologists regarding improvement of treatment efficacy and patients’ desires for minimizing treatment-related toxicities. What if IMRT is unavailable due to practical reasons? No need for guilt, conventional RT is still a legitimately standard treatment. Constant efforts to find the optimal orchestration of the available modalities including EBRT, brachytherapy, systemic therapy, and surgery tailored to individual patient may prove satisfactory.
Notes
Author Contributions
Conceived and designed the analysis: Lee SW, Lee JH.
Collected the data: Lee SW, Kim A, Lee SJ, Kim SH, Lee JH.
Contributed data or analysis tools: Lee SW, Kim A, Lee SJ, Lee JH.
Performed the analysis: Lee SW, Lee SJ, Lee JH.
Wrote the paper: Lee SW, Lee JH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.