Clinical Features of Li-Fraumeni Syndrome in Korea
Article information
Abstract
Purpose
Li-Fraumeni syndrome (LFS) is a hereditary disorder caused by germline mutation in TP53. Owing to the rarity of LFS, data on its clinical features are limited. This study aimed to evaluate the clinical characteristics and prognosis of Korean patients with LFS.
Materials and Methods
Patients who underwent genetic counseling and confirmed with germline TP53 mutation in the National Cancer Center in Korea between 2011 and 2022 were retrospectively reviewed. Data on family history with pedigree, types of mutation, clinical features, and prognosis were collected.
Results
Fourteen patients with LFS were included in this study. The median age at diagnosis of the first tumor was 32 years. Missense and nonsense mutations were observed in 13 and one patients, respectively. The repeated mutations were p.Arg273His, p.Ala138Val, and pPro190Leu. The sister with breast cancer harbored the same mutation of p.Ala138Val. Seven patients had multiple primary cancers. Breast cancer was most frequently observed, and other types of tumor included sarcoma, thyroid cancer, pancreatic cancer, brain tumor, adrenocortical carcinoma, ovarian cancer, endometrial cancer, colon cancer, vaginal cancer, skin cancer, and leukemia. The median follow-up period was 51.5 months. Two and four patients showed local recurrence and distant metastasis, respectively. Two patients died of leukemia and pancreatic cancer 3 and 23 months after diagnosis, respectively.
Conclusion
This study provides information on different characteristics of patients with LFS, including types of mutation, types of cancer, and prognostic outcomes. For more appropriate management of these patients, proper genetic screening and multidisciplinary discussion are required.
Introduction
Li-Fraumeni syndrome (LFS) is a rare autosomal dominant hereditary disorder caused by a germline mutation in TP53 [1,2]. TP53 gene is a tumor suppressor gene that provides instruction for making a protein called p53 and plays a key role in apoptosis, cell cycle regulation, and DNA repair [3,4]. Therefore, LFS is associated with a high risk of early-onset malignancies, such as breast cancer, bone and soft tissue sarcoma, brain tumor, adrenocortical carcinoma, leukemia, gastric cancer, and colorectal cancer. Its lifetime risks of malignancy are ≥ 70% for male and ≥ 90% for female patients [1,5,6].
Owing to the high cancer risk of LFS, several efforts have been made to identify its phenotype and genotype. Although previous studies have reported the mutational characteristics of LFS based on locus-specific databases, including the International Agency for Research on Cancer (IARC) TP53 database (http://p53.iarc.fr/) [6,7] and Universal Mutation Database (UMD) (http://p53.fr/) [8], most of the data originate from Western populations.
In Korea, studies have analyzed 14 patients with nine different types of TP53 mutations [9] and 12 patients with LFS with breast cancer [10]. Other case reports also have described patients with LFS with various types of malignancies [11-13].
However, because of the rarity of LFS, data on its types of mutation, clinical features, and treatment outcomes in Korean patients are limited. This study aimed to evaluate the clinical characteristics and prognosis of Korean patients with germline TP53 mutation.
Materials and Methods
Patients who met the LFS criteria clinically, including the classic LFS criteria and Chompret criteria, or had a suspicious personal or family history underwent genetic counseling. All patients with confirmed germline TP53 mutation at the National Cancer Center, Korea, between 2011 and 2022 were included and retrospectively reviewed. Genetic testing was performed by direct sequencing of 11 exons of TP53 gene or by next-generation sequencing (NGS) of > 22 genes. Data on the age at diagnosis of the tumor, sex, personal history, family history with pedigree (Fig. 1), types of mutation, clinical features, and prognosis were collected.
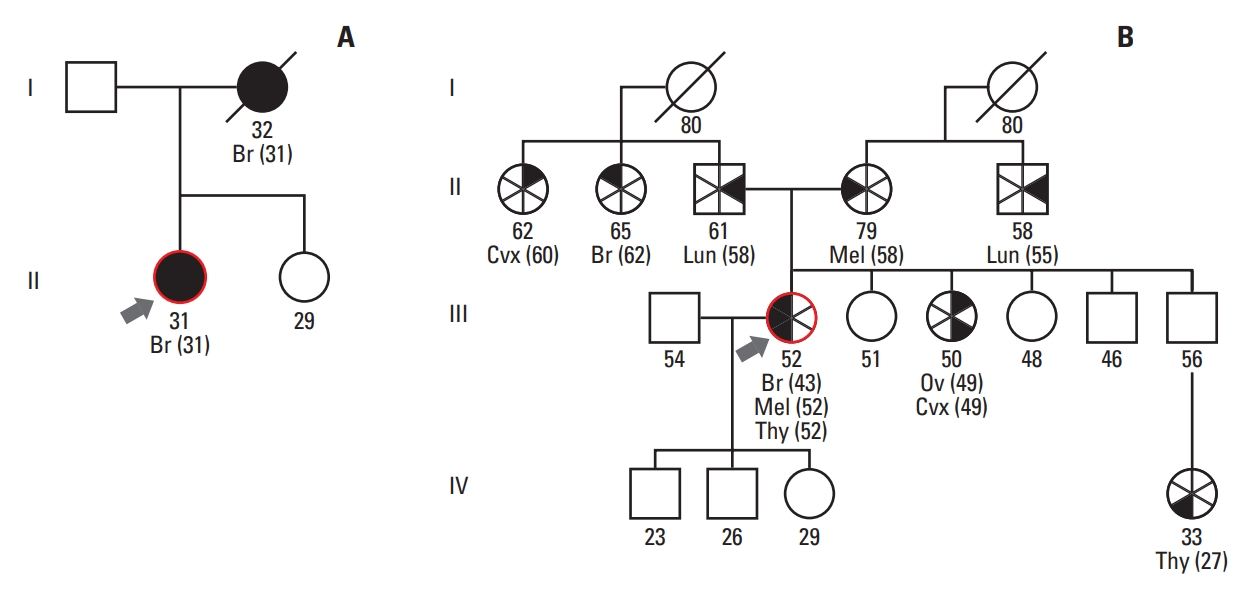
Comparison of pedigrees between patients with Li-Fraumeni syndrome. (A) A pedigree of patient 7 with only a family history of breast cancer. (B) A pedigree of patient 11 with a family history of various malignancies in first- or second-degree relatives and who met the Chompret criteria. Br, breast cancer; Cvx, cervical cancer; Lun, lung cancer; Mel, melanoma; Ov, ovarian cancer; Thy, thyroid cancer.
Results
Fourteen patients with germline TP53 mutation were included in this study. Clinical and TP53 mutation data are summarized in Table 1.
1. Demographic data
Among the total patients, there was only one male patient and the rest were female (male:female ratio, 0.1:1). The median age at diagnosis of the first tumor was 32 years (range, 1 to 67 years). A family history of primary cancer was observed in 12 patients, and family members of eight patients had LFS-related tumors, including osteosarcoma, breast cancer, brain tumor, and lung cancer. Three patients, including two patients who were sisters, had a sibling diagnosed with LFS.
2. Types of germline TP53 mutation
Half of the patients underwent genetic testing using direct sequencing, and the other half underwent multigene panel NGS. Eleven different types of mutation were identified in the 14 Korean patients. Missense and nonsense mutations were observed in 13 and one patients, respectively. The repeated mutations were located at codons 273 (p.Arg273His, n=2), 138 (p.Ala138Val, n=2), and 190 (p.Pro190Leu, n=2) (Fig. 2), and the sister with breast cancer harbored the same mutation of p.Ala138Val. All mutations were heterozygous, including one case of mosaicism (patient 10) with a variant allele frequency of 11.4%.
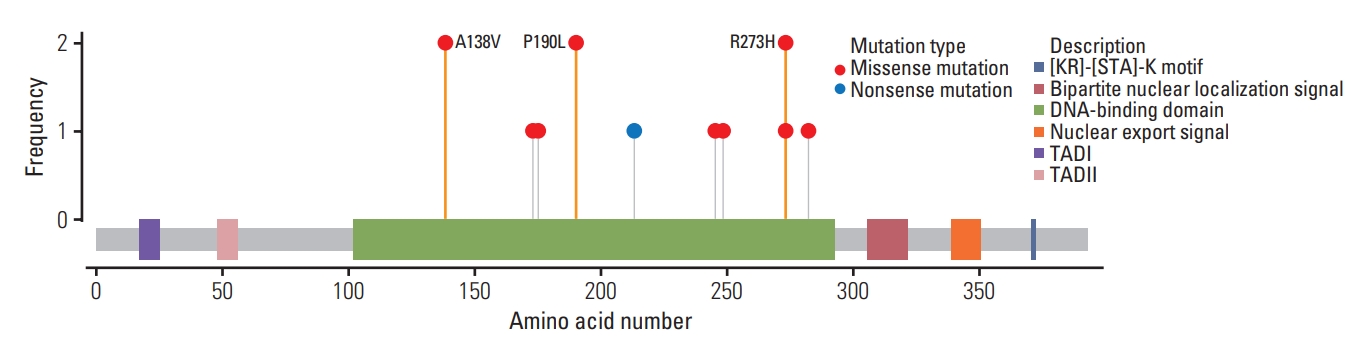
Lollipop mutation diagram of the study population. All mutations shown in this study were at DNA-binding domain. Except for one patient with nonsense mutation in codon 213, other 13 patients showed missense mutations. A138V (n=2), P190L (n=2), and R273H (n=2) were repeated, and two patients with A138V mutation were sisters. A, alanine; H, histidine; L, leucine; P, proline; R, arginine; T, tryptophan; V, valine.
3. Clinical characteristics
Twelve patients had suspicious clinical features, two of whom met the classic LFS criteria and eight met the Chompret criteria. A patient with early-onset breast cancer and a negative BRCA test (patient 7) and a patient with multiple primary tumors (patient 12) also underwent genetic testing. Two patients diagnosed with pancreatic cancer did not have a significant family history in pedigrees, but TP53 mutations were found during screening.
Various primary malignancies were identified. “Core” cancers, including breast cancer, sarcoma, brain tumor, and adrenocortical carcinoma, were observed in 12 patients (85.7%). Breast cancer was most frequently observed (n=9), and other types of tumor included sarcoma (n=5), thyroid cancer (n=3), pancreatic cancer (n=2), brain tumor (n=1), adrenocortical carcinoma (n=1), ovarian cancer (n=1), endometrial cancer (n=1), colon cancer (n=1), vaginal cancer (n=1), skin cancer (n=1), and leukemia (n=1). Seven (50%) patients had multiple primary cancers (range, 2 to 4). Leukemia found in one patient was induced by treatment of the preceding breast cancer (patient 3).
In some malignancies, biomarker testing was performed on cancer specimens for the selection of drug regimens. Except for patients without data, one BRAF-mutated thyroid cancer and one BRCA1-mutated ovarian cancer were identified (S1 Table).
4. Prognostic outcomes
The median follow-up period from the diagnosis of the first tumor was 51.5 months (range, 6 to 188 months). After the diagnosis of LFS with genetic testing, one patient with breast cancer was diagnosed with colon cancer during the work-up for other malignancies (patient 5), and additional new-onset malignancies were found in three other patients during the follow-up. There were two and four cases of local recurrences and systemic recurrences, respectively. Two patients died of leukemia and pancreatic cancer 3 and 23 months after diagnosis, respectively.
Discussion
In this study, we reviewed Korean patients with LFS to evaluate their types of mutation and clinical features. There were 11 different types of mutation in 14 patients, and various spectra of malignancies were found.
Studies based on the IARC p53 database showed that most TP53 mutations were missense mutations, accounting for 74%, and nonsense and splice mutations accounted for approximately 9% and 8%, respectively [6,7]. Except for one patient with nonsense mutation, all patients in this study had missense mutation. Park et al. [9] investigated the mutations and tumor spectra of LFS in Korean patients and identified two novel frameshift mutations in codons 98 and 27. Other studies on Korean patients have reported other types of mutation, such as frameshift or silent mutation (S2 Table) [9-18]. Based on the IARC database, the most common hotspot mutations were found in codons 175, 245, 248, 273, and 282 (http://p53.iarc.fr/). In this study, six different mutations were identified in these hotspots in seven unrelated patients. Other mutations were located in codons 138, 173, 190, and 213. Mutations in codons 173 (p.Val173Leu, p.Val173Met) and 213 (p.Arg213*) have not been reported in the Korean Reference Genome Database (KRGDB) or Genome Aggregation database (gnomAD).
In addition to the types of mutation, the age at onset of the first tumor varied in this study. Studies have reported that age and sex are associated with cancer risk in patients with LFS. Cancer risk is highest after the age of 20 years in female patients, whereas it is higher in childhood and later adulthood in male patients [5]. It is difficult to compare because there was only one male patient in the present study, but compared with the 1-year-old male patient, all female patients, except for two teenagers, were adults. A previous study reported a positive relationship between the age of onset of the first tumor and development of secondary tumors [19]. In this study, secondary malignancies were observed in two of four patients (50%), four of seven patients (57.1%), and one of three patients (33.3%) aged 0-19, 20-44 years, and ≥ 45 years, respectively. This result is due to the relatively small number of patient aged 0-19 years, and two of them had short follow-up periods of 11 and 25 months.
Among the tumors identified in this study, core cancers, such as breast cancer and sarcoma, were frequently observed (85.7%), as reported previously [6,20,21]. Notably, two out of 14 patients were diagnosed with pancreatic cancer. According to the IARC database, the relative risk of pancreatic cancer in patients with LFS is 7.3, with an incidence rate of 1.2% [22]. However, our study showed a higher incidence of pancreatic cancer, even compared to other Korean studies (Table 2). This is because, in our institution, genetic testing was performed for screening all patients with any risk of hereditary pancreatic cancer syndrome. Another difference from previous studies is that no patients with gastric cancer were included, although this study was conducted on Korean patients. A study reported that gastric cancer occurred more frequently in Asian populations than in similar Caucasian cohorts [23]. Other Korean studies have reported the incidence of stomach cancer (Table 2).
As is already well known [20], breast cancer was most frequently identified in this study, with a rate of 64.3%. Three patients had bilateral breast cancer, two of whom had metachronous and one had synchronous breast cancer. In the 12 cases of breast cancer in 9 different patients, the median age at diagnosis was 33.5 years (range, 23 to 47 years). Except for two cases with no pathological information, seven (70%) were endocrine receptor (ER)–positive and six (60%), including three with ER co-expression, were human epidermal growth factor receptor 2 (HER2)–positive, showing a higher rate of HER2 expression than the general population [24]. Breast-conserving surgery with adjuvant radiation therapy was performed in three (25%) cases, and the other nine (75%) cases underwent mastectomy (Table 3).
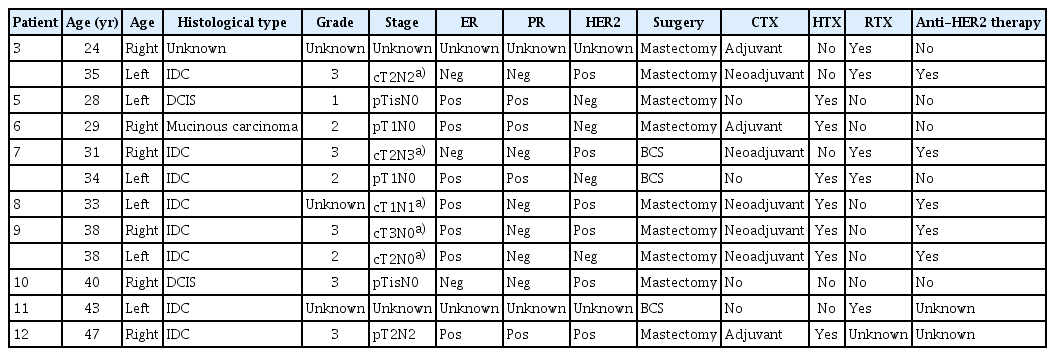
Clinicopathologic features, treatment, and follow-up data in patients with breast cancer with Li-Fraumeni syndrome
These results are similar to those of previous studies investigating the clinicopathological features of breast cancer in patients with LFS [25,26]. Breast cancer showed early onset with a median age of 32-34 years, and in immunohistochemistry staining, the proportion of receptor expression was similar with 84%-90.5% ER positivity, 39.5%-63% HER2 positivity, and 32.8%-53% ER and HER2 co-expression. However, a Korean study by Alyami et al. [10] showed differences in histological features and treatment outcomes. This study reported a lower rate of ER expression and a higher rate of triple-negative breast cancer. The recurrence rate was also higher than that in our study, which may be due to differences in subtypes.
A patient (patient 3) of the present study diagnosed with bilateral breast cancer died of acute myeloid leukemia induced by breast cancer treatment. Similar with this case, several reports demonstrated that therapeutic interventions, such as chemotherapy and radiation therapy, caused therapy-induced secondary malignancies, including breast cancer [27,28], sarcoma [29], thyroid cancer [28,29], central nervous system tumor [28], and leukemia [9,28,30]. Despite clinical suspicion, there have been no large-scale comparative studies supporting the tumorigenesis of therapeutic interventions. Further multicenter studies with larger populations are required to evaluate which treatments can increase the risk of malignancy or which tissues are particularly vulnerable. Clinicians should be aware of the possibility of secondary cancer and should be careful when making treatment decisions.
This study is one of the largest case series performed in Korean patients with LFS and includes prognostic data. However, this study has some limitations. This was a retrospective study, and the data were obtained from medical records. Therefore, clinical information, such as indications for genetic testing or environmental factors, including lifestyle, was insufficient. In addition, this study was conducted in a single institution, and the number of included patients was small; therefore, it is difficult to define the characteristics and prognosis of patients with the disease. Finally, because this study included patients lost to follow-up or recently diagnosed patients, the prognostic outcomes were inaccurate.
This study provides information regarding the mutational features and clinical characteristics of patients with LFS in South Korea. Similar to other countries, several Korean patients with LFS also have early onset and multiple primary tumors. The clinical features and prognosis vary according to the type of mutation, type of cancer, and time of diagnosis.
This study emphasizes that genetic counseling and screening are required not only for patients but also for their family members. And for proper management, clinicians should assess genetic testing results and conduct multidisciplinary discussion before making treatment decisions. Further multicenter, large-cohort studies are required for the proper screening and management of Korean patients with LFS.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the Institutional Review Board of the National Cancer Center, Korea (IRB No. NCC2022-0268). The requirement for informed consent was waived owing to the retrospective nature of this study.
Author Contributions
Conceived and designed the analysis: Song R, Kong SY, Jung SY.
Collected the data: Song R.
Contributed data or analysis tools: Song R.
Performed the analysis: Song R.
Wrote the paper: Song R.
Interpretation of data, critical revision of manuscript, final approval of the version: Song R, Kong SY, Choi W, Jung SY.
Interpretation of data: Lee EG, Woo J, Han JH, Lee S, Kang HS.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
This work was supported by a National Cancer Center Grant (No. NCC 2110181-3).


