Strategies to Improve Smoking Cessation for Participants in Lung Cancer Screening Program: Analysis of Factors Associated with Smoking Cessation in Korean Lung Cancer Screening Project (K-LUCAS)
Article information
Abstract
Purpose
Smoking cessation intervention is one of the key components of successful lung cancer screening program. We investigated the effectiveness and related factors of smoking cessation services provided to the participants in a population-based lung cancer screening trial.
Materials and Methods
The Korean Lung Cancer Screening Project (K-LUCAS) is a nationwide, multi-center lung cancer screening trial that evaluates the feasibility of implementing population-based lung cancer screening. All 5,144 current smokers who participated in the K-LUCAS received a mandatory smoking cessation counseling. Changes in smoking status were followed up using a telephone survey in 6 months after lung cancer screening participation. The lung cancer screening’s impact on smoking cessation is analyzed by variations in the smoking cessation interventions provided in screening units.
Results
Among 4,136 survey responders, participant’s motivation to quit smoking increased by 9.4% on average after lung cancer screening. After 6 months from the initial screening, 24.3% of participants stopped smoking, and 10.6% of participants had not smoked continuously for at least 6 months after screening. Over 80% of quitters stated that participation in lung cancer screening motivated them to quit smoking. Low-cost public smoking cessation program combined with lung cancer screening increased the abstinence rates. The smokers were three times more likely to quit smoking when the smoking cessation counseling was provided simultaneously with low-dose computed tomography screening results than when provided separately.
Conclusion
A mandatory smoking cessation intervention integrated with screening result counselling by a physician after participation in lung cancer screening could be effective for increasing smoking cessation attempts.
Introduction
Lung cancer is the leading cause of all cancer deaths. Almost 25% of all cancer deaths are caused by lung cancer [1]. Following the results of the National Lung Cancer Screening Trial, the U.S. Preventive Services Task Force now recommends annual lung cancer screening with low-dose computed tomography (LDCT) in high-risk individuals, and the recent European Union position statement also recommended planning for the implementation of LDCT screening throughout Europe to reduce lung cancer mortality [2,3]. Recently, a Dutch-Belgian lung-cancer screening trial also has shown that lung cancer screening could reduce lung cancer deaths [4].
The objective of lung cancer screening is to detect lung cancer early thereby improving survival rates, but it is well acknowledged that screening alone may not save as many lives as expected without smoking cessation intervention on screening participants [5]. Smoking still remains the leading cause of lung cancer; thus, the most primitive way to reduce lung cancer mortality is to prevent people from smoking [6].
In this context, lung cancer screening provides an excellent opportunity for smoking cessation intervention as participants in screening are generally followed up for a number of years and are likely to be more concerned with their health than the eligible nonparticipants in screening. It is suggested that lung cancer screening could provide a teachable opportunity for smoking cessation as participation in screening may provoke a greater perception and awareness of potential risk from smoking [7].
Korean Lung Cancer Screening Project (K-LUCAS) which was conducted from 2017 to 2018 to study the feasibility of implementation of population-based lung cancer screening in Korea. In K-LUCAS, all current smokers participating in screening were provided with in-person mandatory smoking cessation counseling and changes in smoking status were followed up 6 months after initial screening. K-LUCAS reported that motivation of lung cancer screening participants to quit smoking increased by 9.2% and the smoking cessation rate was 24.7% at 6-month follow-up based on telephone survey [8].
This study aimed to analyze the factors associated with smoking cessation after participating in the lung cancer screening program. This includes the specialties of physicians providing counseling, whether or not a screening unit is operated by public smoking cessation program, and whether or not counseling is provided simultaneously with screening results.
Materials and Methods
1. Study design and participants
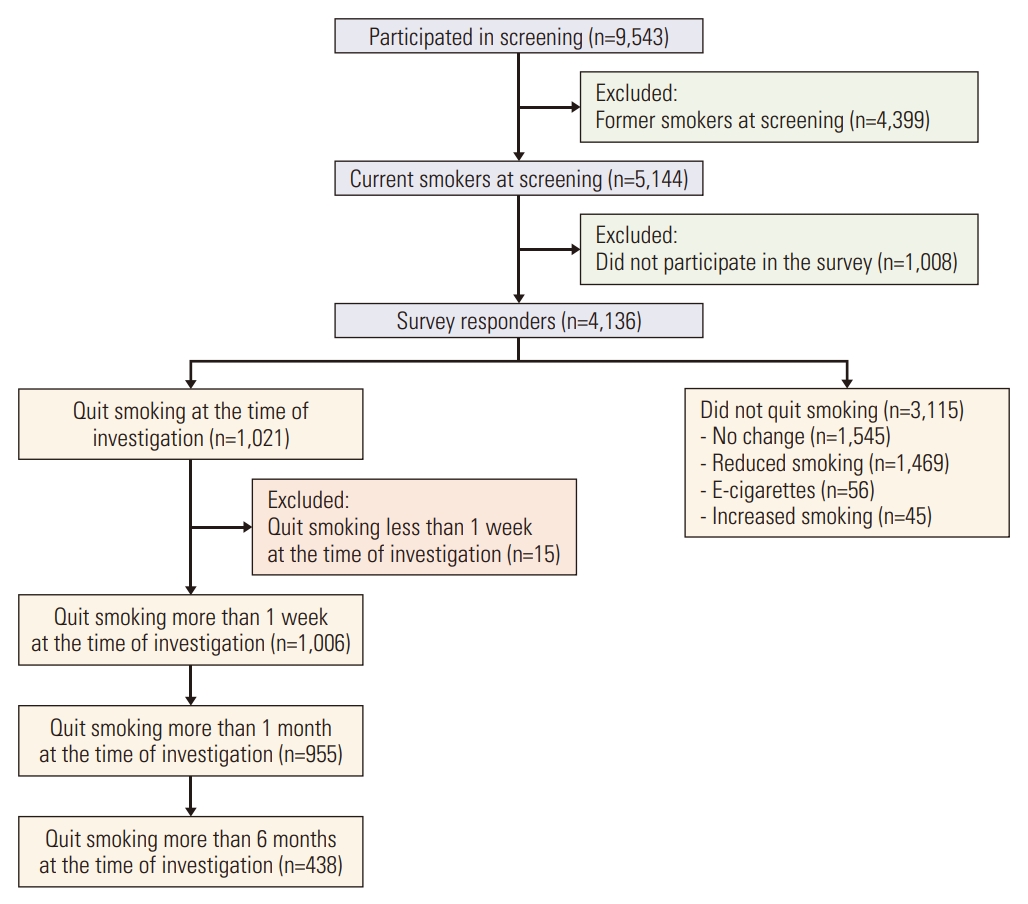
The K-LUCAS was a population-based lung cancer screening trial with LDCT conducted in 14 regional cancer hospitals nationwide. The screening targeted a high-risk group population aged between 55 and 74 years with at least 30 pack-years of smoking history. Participants comprised visitors participating in other national health screening programs or smoking cessation clinics in these hospitals who were previously required to complete a set of questionnaires. Questionnaires included information on age, smoking status, smoking history, and medical history. A detailed description of the study design was reported previously [9]. A total of 9,543 participated in the K-LUCAS between March 2017 and June 2018, of whom 5,144 were current smokers. All current smokers underwent the cotinine test on the day they visited hospital for LDCT screening to verify their current smoking status and were provided with an in-person mandatory smoking cessation counseling on the day when they revisited hospitals to hear LDCT scan results. The K-LUCAS was approved by the institutional review board at each of the 14 participating hospitals and informed consent was obtained from all participants.
2. Smoking cessation intervention
All current smokers in the K-LUCAS were provided with an in-person mandatory smoking cessation counseling but within a different system. Not all hospitals had the same smoking cessation programs. The program details depended on whether the hospital had a public or private smoking cessation program.
In the public smoking cessation program, physicians certified by the Korean Health Promotion Institute performed smoking cessation counseling. Physicians are educated for structured motivational smoking cessation counseling and pharmacotherapy. Physicians performed counseling and provided pharmacological treatments in accordance to nicotine dependence, withdrawal symptom experience, and participant’s preference with the standardized protocol. During the counseling for lung cancer screening results, physicians could register the participants for public smoking cessation programs and prescribe pharmacological treatments, if the participants were willing to quit smoking. The public smoking cessation program provided counselling 6 times by physician with pharmacotherapy within 3 months by low cost. National health insurance covers 70% of the total costs for counseling and medications. If participants successfully attended the program 3 times or more, these out-of-pocket costs were fully reimbursed regardless of smoking cessation results.
Two of fourteen participating hospitals had its own private smoking clinic program with noncertified counseling physicians and unstructured cessation treatments. In privately operated smoking cessation programs, participants pay full cost for smoking cessation counseling and pharmacological treatment. In both programs, if the participants did not object, pharmacotherapy was usually prescribed because lung cancer screening participants were long-term highly addicted smokers.
3. Various approaches to smoking cessation interventions
Some variations in smoking cessation interventions involve 14 screening units. First, the specialty of physicians providing counseling was different between screening units. Smoking cessation counseling was performed by internal medicine doctors in six of the 14 hospitals and by family medicine doctors in the remaining eight hospitals.
Second, the timing of counseling varies. In 12 of the 14 hospitals, the same physician provided smoking cessation counseling simultaneously with LDCT screening results. This allowed physicians to perform counseling with a LDCT scan in hand which could be shown to currently smoking participants. Participants in the other two hospitals received LDCT screening results and smoking cessation counseling separately.
4. Measures of smoking abstinence
Changes in smoking status of all 5,144 current smokers were investigated 6 months after initial screening using a telephone survey. Of the 5,144 current smokers, 4,136 participated in the survey, but 1,008 declined to take part in the telephone survey or we failed to contact them after three attempts. The participants who stated that they had quit smoking for more than 1 week at the point of investigation were accounted for 1-week point abstinence rates, and participants who stated that they had quit smoking for more than 6 months continuously after participating in screening were accounted for continuous abstinence. The 1-week point abstinence rates and 6-month continuous abstinence rates were evaluated in 5,144 current smokers (intention-to-treat analysis) and 4,136 survey responders (per-protocol analysis). Quitters were asked whether or not the participation in lung cancer screening motivated them to quit smoking.
5. Variables and statistical analysis
The logistic regression was used to estimate the effect of variations in smoking cessation intervention on participant’s smoking cessation for 4,136 survey responders. Age, sex, pack-years of smoking history, drinking status, educational level, household income, family history of lung cancer, medical history of lung disease, and screening results were adjusted. Individuals with household income less than 2.0 million Korean won (about 1,600 U.S. dollar) per month were classified as individuals with lower income. Individuals drinking alcohol 5 days per week were classified as heavy drinkers [10]. We coded any unanswered questions as missing data. The LDCT screening results were categorized based on the categories of the Lung Imaging Reporting and Data System (Lung-RADS) ver. 1.0 [11]. Lung-RADS categories 3 and 4 were defined as positive screening results, which required follow-up examination or an additional work-up to rule out lung cancer. Lung-RADS categories 1 and 2 were defined as negative screening results, which do not require further evaluation, but need regular check-up.
The changes in motivation to quit smoking before and after lung cancer screening participation were analyzed using a paired t test. To compare the participants’ general characteristics, a chi-squared test was used. Univariate and multivariate logistic regression models were applied to identify factors associated with smoking cessation. To check the multicollinearity in multiple logistic models, the variance inflation factor (VIF) was assessed how much the independent variables are correlated. An alluvial plot illustrates to show the changes in fraction of smokers’ status at each time points from receiving screening results to smoking cessation. All statistical analyses were performed using the Stata software, ver. 14 (Stata Corp. L.P., College Station, TX).
Results
1. Characteristics
A total of 9,543 participants participated in the K-LUCAS, 5,144 (53.9%) of whom were current smokers. Table 1 presents the summary of the general characteristics of current smokers who participated in the K-LUCAS. Of the 5,144 current smokers, 1,736 (33.8%) were aged older than 65 years. Only 161 current smokers (3.1%) were female. Moreover, 1,306 participants (25.4%) had an education level under middle school, and 2,066 participants (40.2%) had a household income less than 2.0 million Korean won per month. Furthermore, 655 participants (12.7%) were categorized as heavy drinkers. Of the 5,144 current smokers who participated in the K-LUCAS, 822 (16.0%) had positive screening results from the initial LDCT screening.
2. Smoking abstinence rate
Fig. 1 shows the flow of participants in the K-LUCAS and smoking behaviors of current smokers after participating in the screening. A total of 1,021 survey responders stated they had quit smoking at the time of investigation. The 1-week point abstinence rates measured at 6 months after screening participation were 19.6% among 5,144 current smokers and 24.3% among 4,136 survey responders. The 6-month continuous abstinence rates were 8.5% among 5,144 current smokers and 10.6% among 4,136 survey responders.
Of the 3,115 non-quitters, 1,469 (47.2%) stated they had reduced smoking, and only 45 (1.4%) stated that they smoke more after participating in the screening. In total, 2,490 of all current smokers (48.4%) either quit or reduced smoking after participating in lung cancer screening.
Of the 653 participants with positive screening results who were surveyed, 206 (31.5%) stated they had quit smoking for more than 1 week at the point of investigation, and 87 (13.3%) stated they had not smoked continuously for at least 6 months. The 1-week point abstinence rates and 6-months continuous abstinence rates were significantly higher than those of participants with negative findings. The 1-week point abstinence rates and 6-months continuous abstinence rates for participants with negative findings were 23.0% and 10.1%, respectively.
A total of 42 patients were diagnosed with lung cancer during the screening of current smokers. Of these, 12 may have quit smoking but were unable to be surveyed because they were receiving treatments. Of the 30 lung cancer patients who completed the survey, 23 (76.7%) had quit smoking for more than 1 week at the point of investigation, and 15 (50.0%) had not smoked continuously for at least 6 months after initial screening.
3. Motivation of smoking cessation
Overall, the participants’ motivation to quit smoking significantly increased after participating in lung cancer screening. Current smokers’ motivation to quit smoking increased by 9.4% on average, and the same pattern was observed regardless of age, sex, pack-years of smoking history, educational level, household income, and screening results. Of the 1,021 quitters, 848 (83.1%) stated that participation in lung cancer screening motivated them to quit smoking.
4. Various approaches to smoking cessation interventions
Fig. 2 shows the differences in 1-week point abstinence rates at 6 months after screening participation between screening units. The highest and lowest 1-week point abstinence rates reported in a single screening unit were 32.1% and 13.3%, respectively.
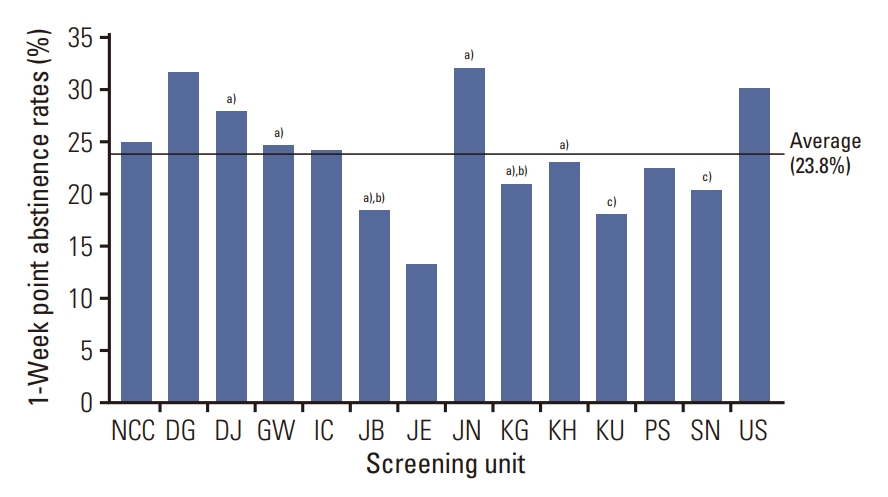
1-week smoking abstinence rates at 6 months after participating in lung cancer screening by screening unit. DG, Daegu-Gyeongbuk Regional Cancer Center; DJ, Daejeon Regional Cancer Center; GW, Gangwon Cancer Center; IC, Incheon Regional Cancer Center; JB, Jeonbuk Regional Cancer Center; JE, Jeju Regional Cancer Center; JN, Jeonnam Regional Cancer Center; KG, Gyeonggi Cancer Center; KH, Kyung Hee University Hospital; KU, Korea University Guro Hospital; NCC, National Cancer Center; NHIS, National Health Insurance Services; PS, Busan Regional Cancer Center; SN, Seoul National University Hospital; US, Ulsan Cancer Center. a)Smoking cessation counseling was performed by internal medicine doctors rather than family medicine doctors, b)Smoking clinics operated privately by a screening unit rather than by the NHIS, c)Counseling was provided separately with low-dose computed tomography screening results.
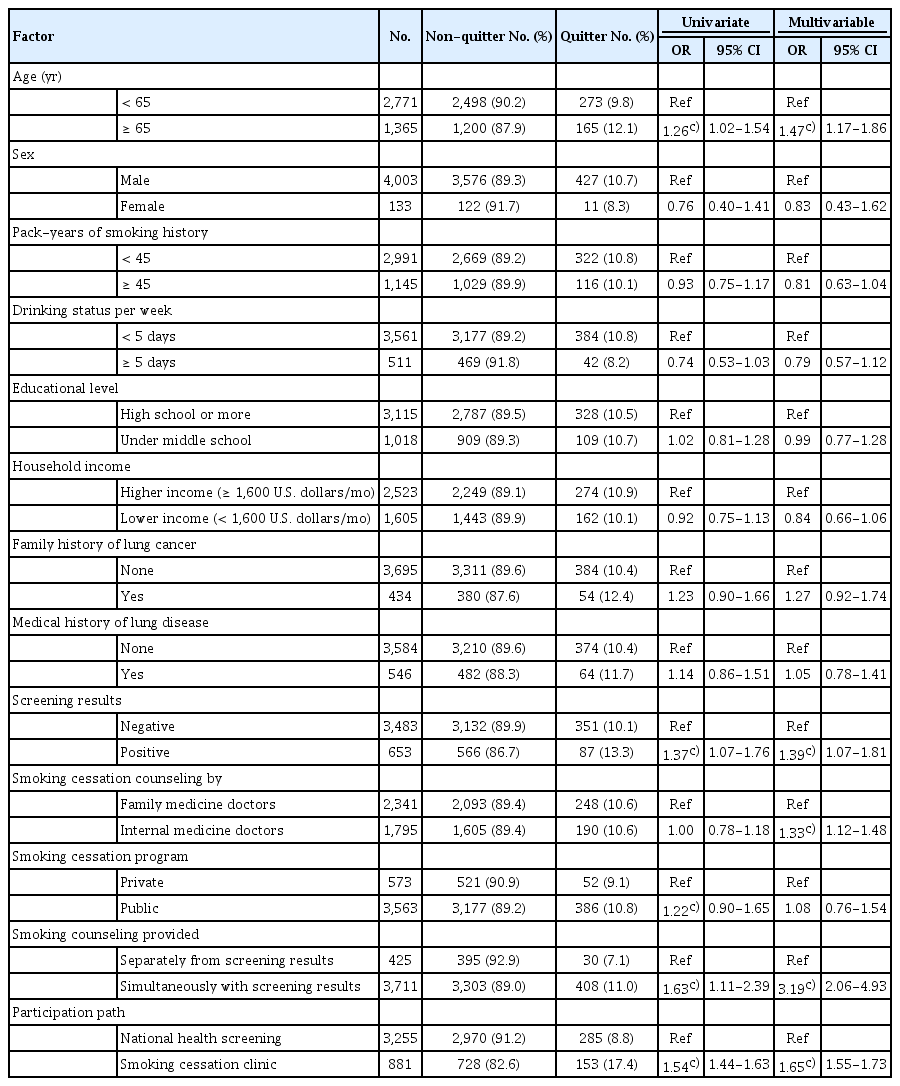
Tables 2 and 3 present the results of logistic regression of variations in intervention on participant’s 1-week point abstinence and on 6-month continuous abstinence, respectively, for 4,136 survey responders. The possibility of the multicollinearity in multiple logistic models is very low, because the mean of VIF was assessed as 1.14.
First, participants who simultaneously received both smoking cessation counseling and LDCT screening results with CT images were 2.0 times (1-week point abstinence [odds ratio (OR), 2.01; 95% confidence interval (CI), 1.50 to 2.70]) or 3.2 times (6-month continuous abstinence [OR, 3.19; 95% CI, 2.06 to 4.93]) more likely to quit smoking than participants who received counseling separately from LDCT screening results. Second, participants who were referred to public smoking cessation programs were 1.3 times (1-week point abstinence [OR, 1.32; 95% CI, 1.03 to 1.69]) more likely to quit smoking than participants who were referred to privately operated clinic programs. Such an effect, however, was insignificant for 6-month continuous abstinence rates. Finally, the specialty of the physician who provided smoking cessation counseling did not have any statistically significant impact on the participant’s point abstinence; however, continuous abstinence rate was higher when participants were counseled by family medicine doctors than by internal medicine doctors.
5. Other factors associated with smoking abstinence
We also found that age and lung cancer screening results were significantly associated with smoking cessation rate. The probability of smoking cessation was higher in older participants than in younger participants. However, family history of lung cancer had no significant difference in quitting smoking in participants undergoing the lung cancer screening program. Participants with positive screening results were 1.6 times (1-week point abstinence [OR, 1.55; 95% CI, 1.28 to 1.87]) or 1.4 times (6-month continuous abstinence [OR, 1.39; 95% CI, 1.07 to 1.81]) more likely to quit smoking than participants with negative screening results. The family history of lung cancer significantly increased the OR of 1-week point abstinence only (OR, 1.29; 95% CI, 1.03 to 1.63).
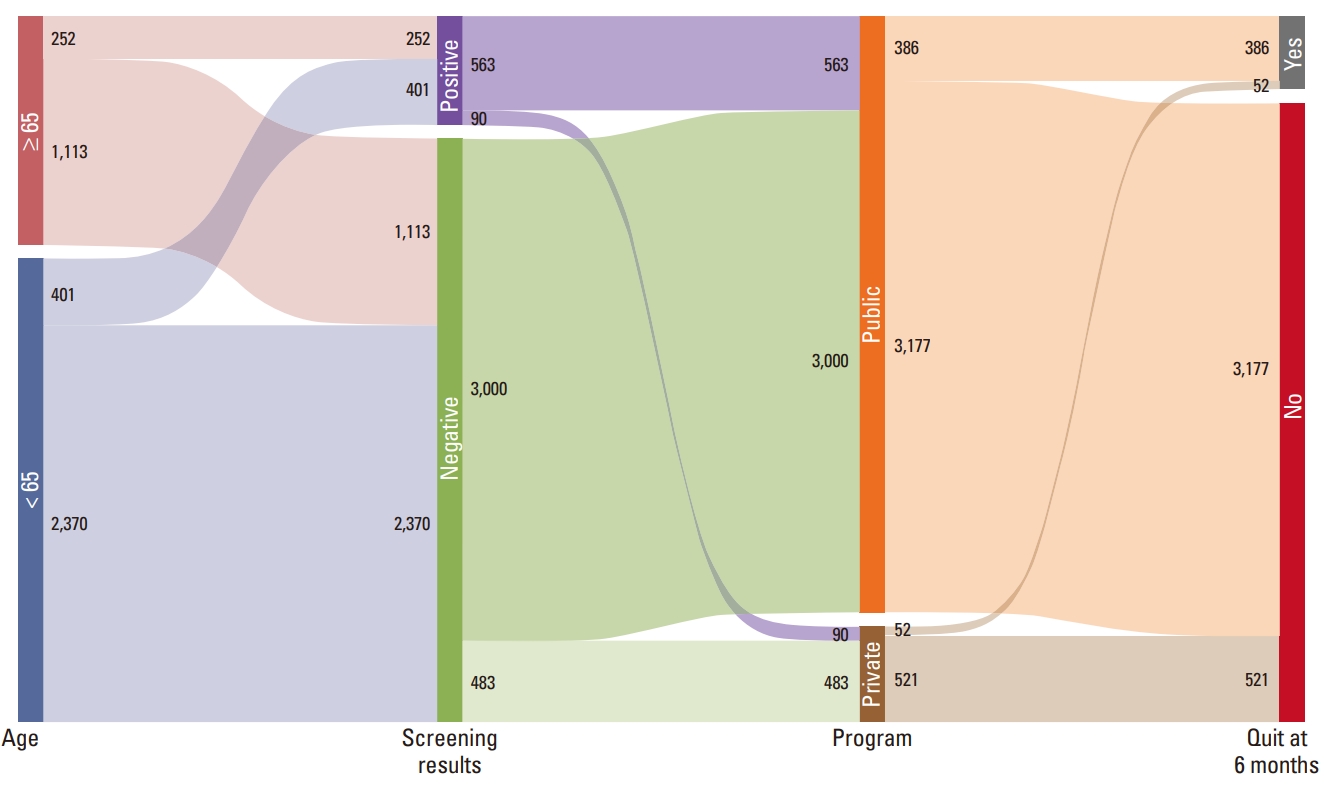
An alluvial plot shows the high proportion of success in continuous smoking cessation after lung cancer screening is the participants who received positive screening results and went public smoking cessation clinic (Fig. 3).
Discussion
Lung cancer screening provides a golden opportunity for smoking cessation intervention as participants in screening are likely to be more concerned with their health than nonparticipants. Lung cancer screening provides a teachable opportunity for smoking cessation as participation in lung cancer screening may provoke a greater perception and awareness of potential risk from smoking [7]. Previous study indicates that an increase of smoking cessation is a very important factor to improve the cost-effectiveness of lung cancer screening, which would be critical in any national screening programs [12]. Previous randomized controlled lung cancer screening trials have indeed shown that smoking cessation intervention incorporated in a lung cancer screening program can be effective in motivating screening participants to quit smoking. The Danish Lung Cancer Screening Trial (DLCST) reported smoking cessation rates of 10.9% at 1-year after baseline screening [13]. The UK Lung Cancer Screening Trial (UKLS) has shown abstinence rates of 23.6% at the 2-years point [14]. In a Dutch-Belgian randomized lung cancer screening trial (NELSON), 14.5% of participants in the LDCT screening arm quit smoking 2 years after baseline screening although this was lower than that of the controlled arm (no screening, 19.1%) [15]. The smoking abstinence rates were 20.8% at 4 years after baseline screening in the Italian Lung Study (ITALUNG) and 12.8% at 2 years after baseline screening in the German Lung Cancer Screening Trial (LUSI) [16,17].
This study has provided further evidence that smoking cessation intervention incorporated in lung cancer screening program can be effective in motivating currently smoking participants to quit smoking, consistent with previous findings. In K-LUCAS, the 1-week point abstinence rates and 6-month continuous abstinence rates were 24.3% and 10.6% respectively, which were much higher than the average smoking cessation rate of 3.8% after increasing tobacco price by 80% in January 2015 in Korea [18]. Based on the survey, more than 80% of quitters in the K-LUCAS stated that participating in lung cancer screening motivated them to quit smoking.
There were some differences between the factors that indicate smoking cessation success measured by 1-week point abstinence and by continuous abstinence after 6 months of lung cancer screening participation. Family history of lung cancer and public smoking cessation program participation are significantly related to higher success in smoking cessation measured by 1-week point abstinence, but not significant in continuous abstinence. Most of the studies regarding smoking cessation reported lower continuous abstinence rates than point abstinence rates [19]. The achievement of continuous abstinence is difficult because every smoking episode after intervention was counted as failure. Thus, the robust indicator of continuous abstinence might not show a significant difference between the factors associated with success of smoking cessation. However, professions providing smoking cessation counselling showed a significant difference in smoking cessation success between family medicine doctors and internal medicine doctors. This phenomenon seems to have occurred because participants who have the will to quit smoking might continue to visit family medicine clinics that mainly provide smoking cessation counseling. Previous studies reported one of the important factors to lead smoking cessation is how many times smokers periodically visited their physicians for quitting smoking [20,21]. It seems that one of the key factor related to success of smoking cessation is not who provides smoking cessation counseling, but how long the care continuity is maintained after initial smoking cessation counseling.
Another interesting finding of this study involves a significant difference in the abstinence rates reported between screening units. The smoking cessation intervention was more effective in some screening units than others. The difference in abstinence rates was as large as 18.8% between the screening units.
Most interestingly, we found that the timing of smoking cessation intervention significantly affects smoking abstinence. Participants who received smoking cessation counseling simultaneously with the LDCT screening results by the same physician were up to 3.2 times more likely to quit smoking than participants who received counseling separately from LDCT screening results. Our results may also partially explain the indifferent (between the control and treatment groups) results in the DLCST where a brief one-to-one smoking counseling is provided separately by trained nurses. In the DLCST, no significant impact of lung cancer screening on smoking cessation was found [13].
A simultaneous provision allowed physicians to provide smoking cessation counseling effectively with images of LDCT scan including pulmonary nodules or emphysema or fibrosis which of smoking related findings could astound current smoking participants. As a consequence of their long history of smoking exposure, it is likely that those participating in the lung cancer screening program would also have smoking-related diseases such as emphysema or coronary artery calcification even though no significant nodules were detected [22,23]. Smoking counseling then might have become more personalized when provided with the screening results or may have provoked greater perception and awareness of risk from smoking as in self-regulation model [24]. Though more personalized smoking cessation incorporated with LDCT results seem to be promising to affect smoking behavior, further study is required to provide data on different approaches of personalized smoking cessation programs incorporated with LDCT results [25].
One potential problem raised in this study is that participants with negative LDCT screening results had significantly lower abstinence rates than participants with positive LDCT screening results, consistent with previous findings [13,26]. Negative screening results could signal false reassurance to smokers to continue smoking. Our results imply that physicians’ counselling with simultaneous provision of LDCT screening results in smoking cessation intervention could prevent negative lung screening results becoming a license to smoke because physicians discussed other findings related with smoking. Also, physicians motivated participants to quit smoking to prevent lung cancer. In our study, about 17.3% of currently smoking participants with negative (no significant nodule detection) screening results had quit smoking when they received smoking cessation counseling and screening results separately, but 23.7% of currently smoking participants with negative results had quit smoking when smoking counseling was performed with LDCT scans (p < 0.01).
We also observed that of the 4,136 current smokers who participated in screening and surveyed, 3,115 (75.3%) still had not quit smoking after smoking cessation intervention (Fig. 1). There could be other confounding factors affecting an individual’s decision to continue smoking other than variations in the smoking cessation interventions and demographics tested in this study. For example, participant’s current stress status (e.g., temporary job loss), willingness to quit, or counselling skills of physicians could also affect smoking cessation, but this was beyond the scope of this study [27,28]. Currently no systematic approach exists for participants who continue smoking after participating in lung cancer screening program, thus, identifying other factors associated with the participant’s decision to continue smoking would help in designing more effective smoking cessation interventions to incorporate in lung cancer screening programs.
This study has several limitations. First, the primary limitation is due to a single-arm cohort study; thus, no control group assessed the between-group effectiveness of smoking cessation intervention. The effectiveness of smoking cessation intervention in the K-LUCAS was evaluated by comparing the abstinence rates in the K-LUCAS with the general abstinence rates in Korea [18] and previously reported abstinence rates in lung cancer screening trials [13-17]. Second, this study did not include missing data in the logistic regression model, which could decrease statistical power to investigate the factors associated with success of smoking cessation. It could be possible that continuous smokers after lung cancer screening are more in non-responders than survey responders. Thus, the current study reported the smoking abstinence rate in 6 months after lung cancer screening both by intention to treat analysis of total current smokers participating in K-LUCAS and by per-protocol analysis of survey responders. We did not give any information on survey purpose before phone calling after 6 months of screening, so it is unlikely that the participants of K-LUCAS did not respond to the telephone survey because they continued to smoke. Third, the study has analyzed the effect of different approaches to smoking cessation intervention at individual-level by logistic regression but hospital-level analysis was not included. This is mainly because no data was available to control for possibly associated factors at hospital-level such as physician experience or hospital accessibility which may weaken the results obtained. Thus, we only included a nominal comparison of smoking cessation rates between participating hospitals. Fourth, all measures of smoking cessation are self-reported from telephone surveys without any biochemical verification such as a cotinine test, mostly because of limited financial resources and difficulties obtaining the verification by revisiting hospital 6 months after screening. Hence, the possibility of self-reported bias of smoking abstinence rates or interviewer bias still remains. However, previous study has shown that self-reported measure is still a valid measure of smoking abstinence and may be more feasible with a large sample size as in this study [29]. Fifth, the efficacy of different kinds of smoking cessation interventions include pharmacotherapy at individual level has not been evaluated. All current smokers in the K-LUCAS received face-to-face smoking cessation counseling by a physician and, if they agreed, were provided pharmacotherapy for quitting smoking.
Although no data was available at individual-level on how long the participants received pharmacotherapy or compliance of medication, a recent systematic review has shown the efficacy of different types of smoking cessation intervention. According to the review, in-person smoking cessation counselling could increase smoking abstinence more significantly than web-based or telephone counselling. Therefore, in-person counselling by physician and actively providing pharmacotherapy could increase smoking cessation more effectively targeting to the high-risk smokers participating in lung cancer screening [30]. Indeed, the smoking cessation rates in K-LUCAS (in-person counseling) were 1.7 times higher than that of the UKLS (brochures with information of smoking cessation) which also targeted high-risk smoking individuals [14].
Both the NELSON trial and UKLS trial did not provide in-person smoking cessation counseling after LDCT screening. Though a direct comparison was not available, the smoking cessation rates of 11.9% at 1 year after baseline screening in the LUSI trial and that of 10.9% at 1 year after baseline screening in the DLCST were lower than 24.3% at 6-months after baseline screening in the K-LUCAS. The DLCST trial provided in-person counselling which was less than 5 minutes by nurses at each annual visit [13]. The LUSI trial provided smoking cessation counselling by trained psychologists but showed no statistical significance in lowering smoking cessation rates [17]. An active smoking cessation intervention by physicians and pharmacotherapy in the K-LUCAS would possibly be a reason for higher smoking cessation rates than the NELSON and UKLS that did not provide any in-person counselling and LUSI trial and DLCST which did not provide physician’s counselling and pharmacotherapy [14,15].
Future studies should investigate the differences in the effectiveness of different kinds of smoking cessation intervention in high-risk population eligible for lung cancer screening so that the most efficient intervention can be provided when lung cancer screening is implemented.
In-person mandatory smoking cessation intervention was effective in motivating lung cancer screening participants to quit smoking. When smoking cessation counseling is provided simultaneously with LDCT screening results and lung images obtained by CT scan, the effectiveness of smoking cessation intervention in lung cancer screening participants could increase. Participating in lung cancer screening could be the best opportunity to motivate smokers to quit smoking. It will be very important to strengthen the motivation of lung cancer screening participants to quit smoking and to continuously help them quit smoking to reduce the incidence and death of lung cancer in the future.
Notes
Ethical Statement
Informed consent was obtained from all study participants after providing them with information regarding the benefits and harms of lung cancer screening. This study was approved by the Institutional Review Board of the National Cancer Center, Korea (IRB No. NCC2016-0255).
Author Contributions
Conceived and designed the analysis: Kim Y (Yeol Kim).
Collected the data: Kim Y (Yeol Kim), Lee E, Lim J.
Contributed data or analysis tools: Kim Y (Yeol Kim), Lee J.
Performed the analysis: Lee J.
Wrote the paper: Kim Y (Yeol Kim), Lee J, Lee E, Kim Y (Yonghyun Kim).
Contributed to critical revision of the manuscript for important intellectual content: Lee CT, Jang SH, Paek YJ, Lee WC, Lee CW, Kim HY, Goo JM, Choi KS, Park B, Lee DH, Seo HG.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control and national health promotion fund, Ministry of Health and Welfare, Republic of Korea (1720310, 1760810-1), a grant from National Cancer Center, Republic of Korea (2210810).