TNM-Based Head-to-Head Comparison of Urachal Carcinoma and Urothelial Bladder Cancer: Stage-Matched Analysis of a Large Multicenter National Cohort
Article information
Abstract
Purpose
Outcome analysis of urachal cancer (UraC) is limited due to the scarcity of cases and different staging methods compared to urothelial bladder cancer (UroBC). We attempted to assess survival outcomes of UraC and compare to UroBC after stage-matched analyses.
Materials and Methods
Total 203 UraC patients from a multicenter database and 373 UroBC patients in single institution from 2000 to 2018 were enrolled (median follow-up, 32 months). Sheldon stage conversion to corresponding TNM staging for UraC was conducted for head-to-head comparison to UroBC. Perioperative clinical variables and pathological results were recorded. Stage-matched analyses for survival by stage were conducted.
Results
UraC patients were younger (mean age, 54 vs. 67 years; p < 0.001), with 163 patients (80.3%) receiving partial cystectomy and 23 patients (11.3%) radical cystectomy. UraC was more likely to harbor ≥ pT3a tumors (78.8% vs. 41.8%). While 5-year recurrence-free survival, cancer-specific survival (CSS) and overall survival were comparable between two groups (63.4%, 67%, and 62.1% in UraC and 61.5%, 75.9%, and 67.8% in UroBC, respectively), generally favorable prognosis for UraC in lower stages (pT1–2) but unfavorable outcomes in higher stages (pT4) compared to UroBC was observed, although only 5-year CSS in ≥ pT4 showed statistical significance (p=0.028). Body mass index (hazard ratio [HR], 0.929), diabetes mellitus (HR, 1.921), pathologic T category (HR, 3.846), and lymphovascular invasion (HR, 1.993) were predictors of CSS for all patients.
Conclusion
Despite differing histology, UraC has comparable prognosis to UroBC with relatively favorable outcome in low stages but worse prognosis in higher stages. The presented system may be useful for future grading and risk stratification of UraC.
Introduction
Unlike urothelial bladder carcinoma (UroBC), clinical course and optimal therapeutic modalities of urachal carcinoma (UraC) are far less understood. UraC, a distinct tumor similarly found within the bladder, is a relatively rare urologic malignancy constituting less than 1% of all bladder masses requiring surgical intervention [1,2]. Known to most frequently occur at the junction between the umbilical ligament and the bladder dome, UraC arises within the urachal remnant, often lacking overt symptoms such as gross hematuria that is found in UroBC. Early diagnosis of the tumor is further complicated by its usual location at the upper portion of the urinary bladder where complete surgical resection is more difficult.
Due to the low overall incidence worldwide, prognosis of UraC is poorly described in literature with variable findings [3]. A populational comparison of UraC versus non-UraC tumors from the Surveillance, Epidemiology, and End Results (SEER) database found favorable outcomes even after adjusting for various clinio-pathologic factors [4], whereas no significant differences were observed for disease-free survival from a small cohort of 31 UraC subjects [5]. In metastatic settings, UraC seems to confer better outcome, with 68% 5-year cancer-specific survival (CSS) compared to 49% in non-UraC tumors [6]. A more recent analysis of Chinese subjects showed no difference in overall survival (OS) (p=0.921) [7]. Lack of a universally appliable staging system that can be transferred to other non-urachal tumors prevents sufficient comparisons of survival, and although Sheldon [8] and Mayo [9] staging allows 8- and 4-tier respective categories for estimation of prognosis, no single system has been fully validated for superior performance [10]. Therefore, in an attempt to elucidate prognosis and clinical outcome, we reassessed 203 UraC cases based on a modified TNM staging system and compared the findings to 373 UroBC patients.
Materials and Methods
1. Study population and data collection
Collection of UraC patient information and clinical variables was conducted as part of a Korean Urological Oncology Group–Bladder Cancer Research Society initiated project via full-scale survey method. Between January 2000 to December 2018, medical records from 203 UraC patients were gathered from 19 institutions and retrospectively reviewed, with survival information most recently updated at time of data collection. Various methods of treatment ranging from partial to radical cystectomy with or without endoscopic resection were selected at the surgeon’s discretion. All appropriate institutional review board (IRB) approval for individual participating organizations were obtained prior to study initiation and provided written informed consent.
Surgical outcomes for UroBC patients were collected from a single, high-volume tertiary hospital (IRB B-2001-586-113). As most patients treated for UraC received complete en-bloc surgical excision rather than transurethral resection (TUR) alone, UroBC patients who received radical cystectomy (RC) rather than TUR-BT (bladder tumor) were included for adequate comparison. Total of 373 UroBC subjects from 2003 to 2020 were retrospectively reviewed and included in final analysis.
2. Stage matching (Sheldon to TNM staging system)
While no validated staging system for UraC exists, previous literature conventionally report findings based on the Sheldon and Mayo staging systems, which have comparable performance of estimating prognosis [9]. We adopted the Sheldon staging system for evaluation of UraC, as it provided a more elaborate classification for extent of tumor involvement into an 8-category system [11,12]. Similar to the modified TNM staging previously presented in a Mayo Clinic study [13], Sheldon I was matched to UroBC T1 (confined to mucosa), II to T2 (localized), IIIA-C to T3 (regional, advanced), IIID to T4 (extravesical invasion), IVA to N1–2 (nodal invasion) and IVB to M1 (distant metastasis) (S1 Table).
3. Statistical analyses
Demographic data were presented as mean±standard deviation. Baseline characteristics including age, sex, body mass index (BMI), American Society of Anesthesia (ASA) score, smoking history, history of diabetes mellitus (DM), and hypertension (HTN) were collected. Recurrence-free survival (RFS), CSS, and OS were calculated using Kaplan-Meier survival analyses with log-rank tests. To assess predictive factors for each survival outcome, uni- and multivariate Cox proportional hazard regression analyses were performed. All statistical analyses were performed using the SPSS package ver. 24.0 (IBM Corp., Armonk, NY).
Results
1. Baseline characteristics of UroBC and UraC patients
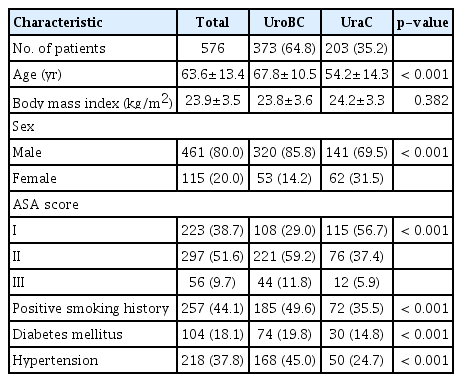
Among total 576 patients, 373 UroBC (64.8%) and 203 UraC (35.2%) patients were included in final analyses. Patients with UraC were significantly younger than those with UroBC (mean age, 54.2 vs. 67.8; p < 0.001, respectively), with higher rate of female patients (31.5% vs. 14.2%, p < 0.001) although patients were predominantly male (Table 1). UroBC was significantly more likely to harbor comorbidities, with 19.8% with DM and 45.0% with HTN in the UroBC group, compared to 14.8% and 24.7% in the UraC group, respectively (both p < 0.001). UroBC also had a higher rate of positive smoking history (49.6% vs. 35.5% in UraC, p < 0.001), as well as worse overall physical status based on ASA classification (p < 0.001).
2. Perioperative and pathological outcomes
Among a total of 203 patients diagnosed as UraC, 163 patients (80.3%) received partial cystectomy (PC) and 23 patients (11.3%) received RC (Table 2). Of the entire cohort, 191 patients (33.2%) received robotic PC or RC. Surgical method was not available in three patients. Operation time was longer in UroBC groups than UraC group (385.6 vs. 173 minutes, p < 0.001), most likely due to the higher rate of extensive lymph node dissection (LND) (92.5% in UroBC vs. 23.2% in UraC) as well as more radical surgery undertaken in UroBC groups. As such, extracted lymph node (LN) count was higher in UroBC group than UraC group (13.7 vs. 3.4, p < 0.001) among patients who received LND. Ultimately, UroBC had more operative blood loss (mean, 839.6 vs. 220.8 mL, p < 0.001) and resulting rate of transfusion (27.3% vs. 5.9%, p < 0.001).
Among 203 UraC subjects, Sheldon stage I, II, IIIA, IIIB, IIIC, IVA, and IVB tumors observed in 11 (5.4%), 32 (15.8%), 6 (3.0%), 87 (42.9%), 38 (18.7%), 8 (3.9%), 4 (2.0%), and 17 (8.4%) patients, respectively. After converting UraC Sheldon stage to appropriately matched TNM stage in UroBC, 5.4% of UraC patients were defined as pT1 or less, 15.7% as pT2, 64.5% as pT3, and 14.3% as pT4 or higher. Rate of advanced, metastatic tumors (pT4 or N1–2 or M1) were similar in both groups (14.2% and 14.3% in UroBC and UraC, respectively). Rate of lymphovascular invasion (LVI) was higher in UroBC group than UraC group (p < 0.001).
Serum biomarkers carcinoembryonic antigen and carbohydrate antigen 19-9 were elevated in 89.7% and 94.6% of UraC cases, respectively. UraC manifestated as adenocarcinoma in 90% of UraC, whereas UroBC was uniformly urothelial carcinoma (transitional cell carcinoma). Invasive squamous cell carcinoma (keratinizing type), nested variant, mucinous neoplasm, and plasmacytoid subtypes were identified in one patient each for UroBC (S2 Table). High-grade UroBC was identified in 84%, with squamous differentiation found in 12%.
3. Survival outcome analysis
During a median follow-up period of 32 months, disease recurrence occurred in 125 (33.5%) patients among UroBC patients and 65 (32.0%) patients among UraC. Cancer-related mortality was reported in 75 (20.1%) UroBC and 34 (16.7%) UraC patients. All-cause mortality was observed in 107 (28.7%) UroBC and 45 (22.2%) UraC patients.
At Kaplan-Meier analysis, 5-year RFS, CSS, and OS were estimated 61.5%, 75.9%, and 67.8% in UroBC and 63.4%, 67%, and 62.1% in UraC, respectively (Fig. 1A–C). Survival outcomes were not significantly different between the two groups in terms of RFS (p=0.570), CSS (p=0.566), and OS (p=0.697).

Recurrence-free survival (A), cancer-specific survival (B), and overall survival (C) comparison of urachal carcinoma (UraC) and urothelial bladder cancer (UroBC).
Stage-matched survival analysis is shown in Fig. 2 after TNM stage conversion. With the exception of CSS in group of pT4 or higher (p=0.028), RFS, CSS, and OS were all similar between the two groups with no statistically significant differences. However, RFS, CSS, and OS all showed relatively favorable trends for UraC in lower stages and unfavorable outcomes for UraC in higher stages compared to UroBC, regardless of p-value. Calculated p-values for RFS, CSS, and OS after log-rank tests were 0.263, 0.261, and 0.308 in pT1 or less; 0.098, 0.899, and 0.957 in pT2; 0.818, 0.552, and 0.123 in pT3; and 0.411, 0.028 and 0.081 in pT4 or higher, respectively. Five-year CSS for pT1, pT2, pT3, and pT4 or higher UraC were 100%, 85.1%, 55.5%, and 16.4%, respectively.
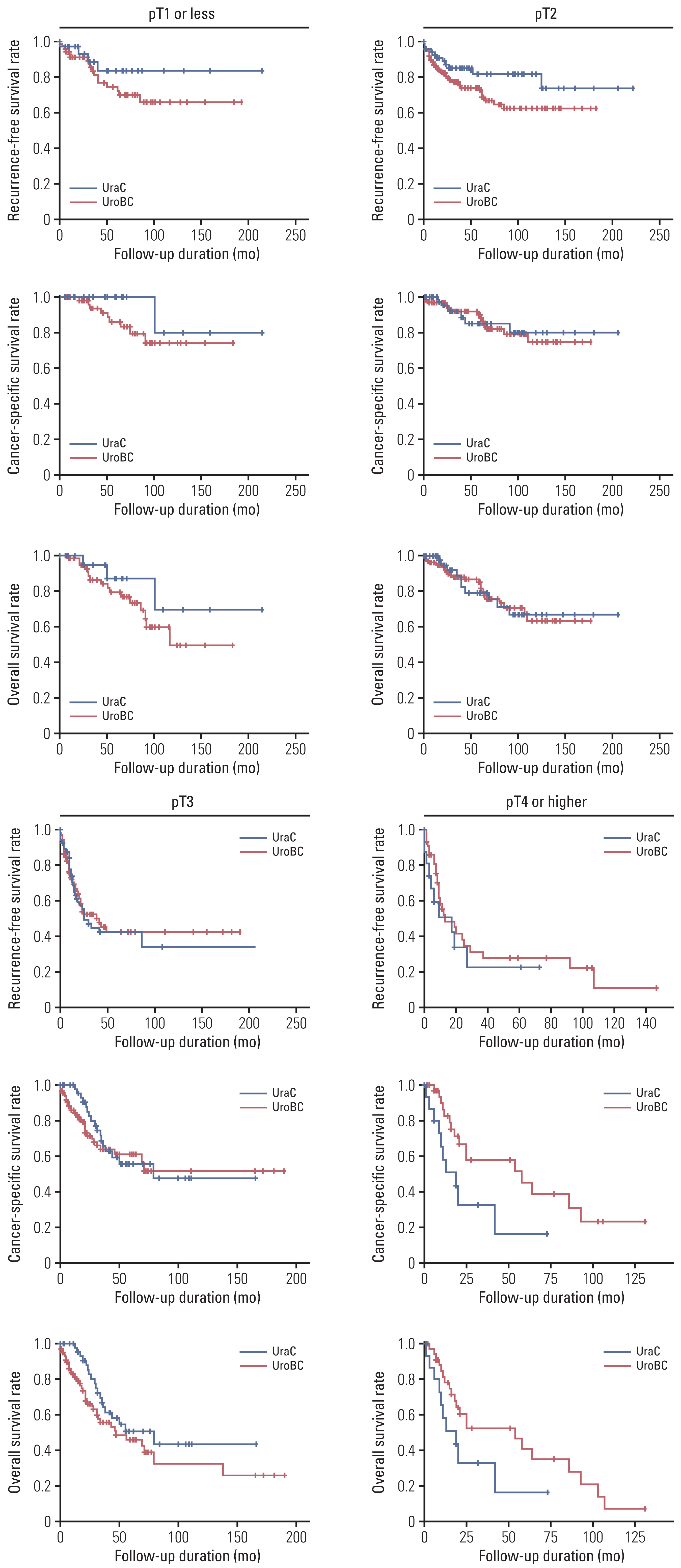
Recurrence-free survival, cancer-specific survival, and overall survival comparison of urachal carcinoma (UraC) and urothelial bladder cancer (UroBC) stratified by matched TNM staging.
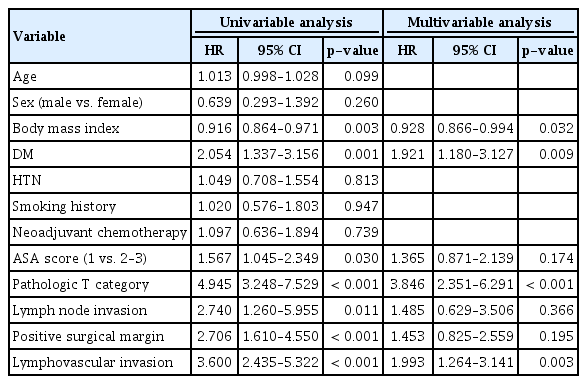
In univariate analysis, BMI (hazard ratio [HR], 0.916; p=0.003), DM (HR, 2.054; p=0.001), ASA score (HR, 1.567; p=0.030), pathologic T category (HR, 4.945; p < 0.001), LN invasion (HR, 2.740, p=0.011), positive surgical margin (HR, 2.706; p < 0.001), and LVI (HR, 3.600; p < 0.001) were significant for CSS among all patients including UroBC and UraC (Table 3). After multivariate analysis, BMI (HR, 0.929; 95% confidence interval [CI], 0.866 to 0.994; p=0.032), DM (HR, 1.921; 95% CI, 1.180 to 3.127; p=0.009), pathologic T category (HR, 3.846; 95% CI, 2.351 to 6.291; p < 0.001), and LVI (HR, 1.993; 95% CI, 1.264 to 3.141; p=0.003) were identified as common predictors of CSS.
Discussion
In this retrospective review of a large multicenter UraC cohort and comparison to UroBC RC outcomes, similar rate of survival was observed between groups for 10-year RFS, CSS, and OS. After stage matching for TNM based on tumor locoregional invasion and metastatic status, however, RFS, CSS, and OS all displayed favorable trends for UraC in pT1/2, with more unfavorable trends in pT3/4 or higher. When further stratified by TNM, UraC showed significantly worse prognosis in pT4 or higher groups for CSS compared to UroBC (p=0.028), with 5-year UraC CSS at 16.4%. Pathologic T category (HR, 3.846; p < 0.001) and LVI (HR, 1.993; p=0.003) were independent predictors of CSS in all patients. The prognostic comparisons of UraC and UroBC in this study suggest that despite differing histology, the two types of tumors have comparable prognosis when matched by TNM. Also, the presented TNM system may be useful for future grading and risk stratification of UraC.
Characterized by its atypical mode of presentation, UraC is caused by the remnant urachus or cystic structures that persist without degeneration to form the median or urachal ligament [14]. Surgical resection of the primary tumor via partial or radical cystectomy remains the mainstay of treatment, although no standardized recommendation exists, mainly due to the sparsity of cases worldwide [15]. In our study, almost all patients received partial rather than RC for UraC; on the other hand, all UroBC patients received RC as per guideline recommendations. Due to the nature of this multicenter study of a relatively rare disease, there was a wide variation in the number of cases collected from each of the 19 organizations, some as few as three cases to as many as 18 cases (mean, 10.7). For UraC, the majority underwent PC (89%) with or without additional TUR-BT, 66% (133) in an open approach, whereas 34% (n=70) underwent minimally invasive laparoscopic or robotic treatment. While PC was generally preferred, choice of surgical modality differed by availability. A high robotic surgery center underwent robotic surgery in 13/18 cases, whereas a low volume center underwent open PC for three out of three cases. Mode of tumor excision, however, did not affect patient outcome in UraC (p=0.397 for OS).
Evidence for the necessity of LND is relatively lacking, with a previous study suggesting that LND does not affect overall outcome [16], unlike in UroBC where maximal lymphadenectomy is crucial for both patient prognosis and optimal staging [17]. While pelvic LN recurrence seems to adversely influence OS, node positivity at initial staging was not a prognostic factor [18]. Results from our study are in line with these reports, as LND in UraC did not affect survival outcome for RFS, CSS, nor OS (p=0.102, p=0.488, and p=0.505, respectively). However, in all patients, LVI independently predicted CSS with HR 1.993 (p=0.003) and was also significant in UraC alone (HR, 4.561; p < 0.001) despite significantly higher rates of LVI in UroBC, suggesting that the role of LND and adequate levels of pelvic LND must be further evaluated.
No single established staging system has been prospectively validated for UraC. The modified TNM staging for UraC, first introduced by Molina et al. [13], provides a much universal categorization for bladder cancer that allows stage comparison to different tumor types, in contrast to the Sheldon system that classifies UraC into eight categories [8]. The TNM staging has the benefits of separating LN involvement and distant metastasis that allow a detailed stratification of risk that is more widely accepted for other malignancies. Similar to the previous TNM staging suggested for UraC [13], Sheldon I, II, IIIA-C, IVA-B tumors were grouped to T1, T2, T3, and T4/N1–2/M1, respectively. However, we categorized Sheldon IIID as T4 which included tumors that extend to visceral structures other than the bladder, for sake of comparison as well as better correspondence to the pT4 definition for UroBC (tumor invading the prostatic stroma, seminal vesicle, uterus, vagina, pelvic wall, or abdominal wall). Results from this method of stratification showed levels of prognosis discrimination comparable to previous literature [9,16], with 100%, 85%, 56%, and 16% 5-year CSS observed for pT1, T2, T3, and T4/N1+/M1 tumors, respectively, while allowing adequate comparison to the conventional staging system for UroBC, suggesting that perhaps a more simplified universal approach to UraC is possible without sacrificing prognostic performance.
The uniform staging in this study allowed head-to-head comparison between the two types of tumors that invade the same organ and are known to have distinctly different cancer characteristics [19]. The asymptomatic presentation of UraC seems to result in higher stages at diagnosis, with nearly 79% of UraC patients staged as T3 or higher at final pathology compared to 42% in UroBC. A previous comparison of 151 UraC and 1,374 UroBC based on the SEER database support these findings [4], with only 20% of UraC harboring localized disease compared to 32% in UroBC. Low-stage UraC patients (pT1 or less) displayed better survival than UroBC, whereas high stage, metastatic UraC tumors tended to have worse prognosis. Earlier studies have found similar or better survival in UraC overall regardless of staging, even for distant disease [3,4]. However, Wright et al. [4] compared UraC to non-UraC, with patients most commonly treated with TUR (55%) rather than cystectomy, potentially resulting in an incomplete resection of histologically diverse tumors in the non-UraC group that may have resulted in a far worse outcome with a median 8-month survival in patients with distant metastasis. Another study of 46 UraC and 106 UroBC patients showed better survival in UraC overall and when matched to similar stages [20]; however, the small sample limit adequate analysis. In our study, stage-matched pT4/N1+/M1 UraC tumors had significantly unfavorable CSS compared to UroBC (p=0.028), suggesting that contrary to previous studies, aggressive UraC may fare far worse outcome compared to UroBC when appropriately matched, despite near identical rate of ≥ T4 (14.3% vs. 14.2% in UraC and UroBC, respectively). Favorable prognosis in early stages may be related to the more radical intervention in UroBC immediately after identification of muscle-invasive tumors (≥ T2) at initial TUR-BT, as well as uniformly younger age at diagnosis for UraC (p < 0.001) with lower rate of comorbidities such as diabetes or HTN (both p < 0.001) and better overall health as evident in lower ASA scores (p < 0.001). While UroBC patients had longer operation times and subsequently higher estimated blood loss and transfusion rates, this is because more radical surgical methods and LND are involved in UroBC and is unrelated to the course of the disease.
In adjuvant settings for recurrent or high-stage tumors in UraC, 33% (n=66) patients subsequently underwent further therapy after initial surgical resection, with the predominant patients receiving chemotherapy (94%, n=64), one with chemotherapy with radiotherapy, and one with radiotherapy only. Cisplatin-based combination therapy with either 5-fluorouracil or gemcitabine were most commonly administered in 22 and 14 patients, respectively. However, other regimens such as FOLFOX (folinic acid-fluorouracil-oxaliplatin) or MVAC (methotrexate-vincristine-doxorubicin-cisplatin) were also used at varying frequencies. For UroBC, 38% (n= 142) underwent adjuvant therapy after primary surgical treatment, with 128 patients receiving platinum-based chemotherapy. Others included nine receiving radiation therapy and five combined therapy.
Our study is limited by its retrospective design and lack of standardized treatment procedures, as well as a relatively small sample size due to the rarity of disease. While no significant differences were observed when matched by pathologic TNM staging, this may be due to the sheer lack of statistical power from limited samples. Also, while the modified TNM grading system in our study incorporates a more comparable approach to conventional staging in other cancers, external validation with outside cohorts should be undertaken to verify its utility. In addition, whether patients received neoadjuvant therapy was not assessed. In addition, only surgically treated UroBC was included, and thus may not fully represent the disease progression in patients treated with other modalities such as tri-modal therapy. Nonetheless, we utilized one of the largest cohorts of UraC and compared the clincopathologic findings to a well-conditioned, single-institution UroBC cohort uniformly treated with RC. TNM-based comparative analyses from this study allow accurate prognosis estimation of UraC and may potentially assist patient counseling and treatment as well as risk stratification.
We compared perioperative and clinicopathological outcomes of UraC and UroBC. While UraC reports generally favorable outcome compared to UroBC, patients are more likely to have non-organ-confined tumors at diagnosis and may have worse prognosis in advanced, metastatic stages when matched by TNM. BMI, presence of DM, matched pathologic staging, and LVI were independent predictors of CSS among all patients. Further large scales studies as well as prospective trials are required to validate our results.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the SNUBH Institutional Review Board (IRB B-2001-586-113). All appropriate institutional review board (IRB) approval for individual participating organizations were obtained prior to study initiation and provided written informed consent.
Author Contributions
Conceived and designed the analysis: Song SH, Oh JJ.
Collected the data: Song SH, Lee J, Ko YH, Kim JW, Jung SI, Kang SH, Park J, Seo HK, Kim HJ, Jeong BC, Kim TH, Choi SY, Nam JK, Ku JY, Joo KJ, Jang WS, Yoon YE, Yun SJ, Hong SH, Oh JJ.
Contributed data or analysis tools: Song SH, Ko YH, Kim JW, Jung SI, Kang SH, Park J, Seo HK, Kim HJ, Jeong BC, Kim TH, Choi SY, Nam JK, Ku JY, Joo KJ, Jang WS, Yoon YE, Yun SJ, Hong SH, Oh JJ.
Performed the analysis: Song SH, Lee J, Oh JJ.
Wrote the paper: Song SH, Oh JJ.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This research was supported by the SNUBH research fund (No. 02-2021-0031) and the National Research Foundation of Korea (NRF 2020R1A2C1100011).