Efficacy of Olaparib in Treatment-Refractory, Metastatic Breast Cancer with Uncommon Somatic BRCA Mutations Detected in Circulating Tumor DNA
Article information
Abstract
Poly(ADP-ribose) polymerase inhibitors have been shown dramatic responses in patients with BRCAness. However, clinical studies have been limited to breast cancer patients with germline mutations. Here, we describe a patient with metastatic breast cancer who had a rare BRCA1 somatic mutation (BRCA1 c.4336G>T (p.E1446*)) detected by cell-free DNA analysis after failing standard therapies. This tier III variant of unknown significance was predicted to be a pathogenic variant in our assessment, leading us to consider off-label treatment with olaparib. The patient responded well to olaparib for several months, with a decrease in allele frequency of this BRCA1 somatic mutation in cell-free DNA. Olaparib resistance subsequently developed with an increase in the allele frequency and new BRCA1 reversion mutations. To our knowledge, this is the first report confirming BRCA1 c.4336G>T (p.E1446*) as a mutation sensitive to olaparib in breast cancer and describing the dynamic changes in the associated mutations using liquid biopsy.
Introduction
Poly(ADP-ribose) polymerase inhibitors (PARPi) are a group of drugs that target the DNA repair pathway and cause synthetic lethality in susceptible cancer cells. Two PARPi monotherapies—olaparib and talazoparib—are approved for use in patients with human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer harboring deleterious germline BRCA mutations. However, the effectiveness of PARPi for breast cancer carrying somatic BRCA mutations has not been established, as most clinical studies have been restricted to patients with harmful germline BRCA mutations [1,2]. Recent research suggests that PARPi may also be effective in breast cancer patients with somatic BRCA1/2 mutations [3], but there is insufficient evidence in actual patients, especially in those with tumors containing rare mutations of unknown clinical significance.
In this case report, we describe a patient with metastatic breast cancer with the rare somatic mutation BRCA1 c.4336G >T (p.E1446*), who exhibited a clinical response to olaparib. The presence of this mutation has been reported in ovarian cancer, but the clinical implications of this mutation, including the response to PARPi, are unknown [4]. Using serial assays of circulating tumor DNA (ctDNA) obtained by liquid biopsy, we were able to track the response to olaparib and detect the development of resistance. We identified reversion mutations in BRCA1 reflecting additional mutations leading to recovery from BRCAness, which has been previously proposed as one of the mechanisms of acquired resistance to PARPi [5,6].
Case Report
1. Patient
Written informed consent was obtained from the patient to publish this case report, and the protocol was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB number: H-1805-049-944), in accordance with the Declaration of Helsinki regarding biomedical research involving human subjects.
2. Sample collection and DNA extraction
Peripheral blood samples (20 mL) were collected into AlphaLiquid Tubes (IMBdx, Seoul, Korea) between June 2021 and July 2022 (Fig. 1A). Plasma was separated by centrifugation with Ficoll solution at 1,500 ×g for 15 minutes and then centrifuged at 16,000 ×g for 10 minutes to remove cell debris. Cell-free DNA (cfDNA) was isolated from 2–4 mL of plasma using a Maxwell RSC cfDNA Plasma Kit (Promega, Madison, WI), according to the manufacturer’s instructions. Genomic DNA was isolated from peripheral blood mononuclear cells using a Maxwell RSC Blood DNA Kit (Promega).
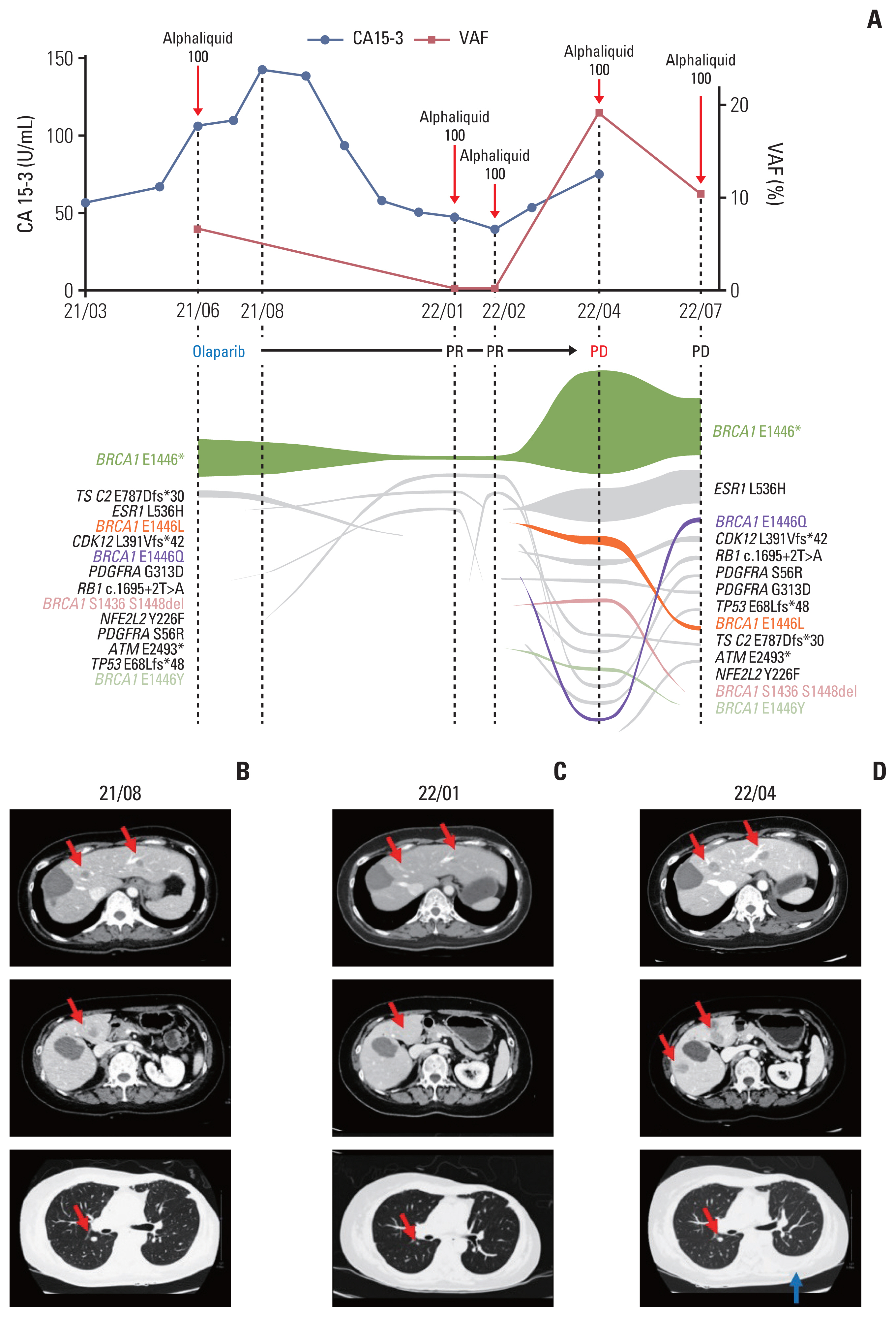
Circulating tumor DNA (ctDNA) analysis and the response to ctDNA-based therapy. (A) Temporal changes of cancer antigen 15-3 (CA 15-3) levels and variant allele frequencies of BRCA1 p.E1446* with ctDNA tumor response map. (B, C) Computed tomography (CT) scans before and during olaparib treatment showed significant shrinkage of the liver and lung nodules (red arrows) during treatment. (D) A follow-up CT scan showed increasing size of the liver and lung nodules (red arrows) and the presence of a new left pleural effusion (blue arrow). PD, progression of disease; PR, partial response; VAF, variant allele frequency.
3. Next-generation sequencing and analysis
AlphaLiquid 100 target capture panel (IMBdx) and IMBdx NGS Library Prep Kit (IMBdx) were used for solution-based target enrichment and next-generation sequencing library construction, respectively. Captured DNA libraries were sequenced using the NovaSeq 6000 platform (Illumina, San Diego, CA) in 2×150 bp paired-end mode. Trimmed reads were aligned to the human reference genome (hg38) using BWA (ver. 0.7.10), and mutation calling was performed using our in-house algorithm (manuscript in preparation).
4. Data availability
The datasets supporting the findings of this report are not publicly available to protect patient privacy. The data will be made available on reasonable request to the corresponding authors.
5. Case presentation
A 48-year-old woman presented with a new palpable left breast mass in December 2007. Biopsy revealed an estrogen receptor–positive, progesterone receptor–positive, and HER2-negative (immunohistochemistry 1+) invasive ductal carcinoma. Initial imaging showed no evidence of nodal involvement or distant metastasis (clinical stage cT3N0). She was treated with a left modified radical mastectomy, at which time the pathologic stage was pT3N2. The patient received adjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by paclitaxel and radiation therapy to the chest wall and regional nodes. She then began endocrine therapy with tamoxifen for a planned duration of 10 years.
After 7 relapse-free years, a surveillance bone scan in March 2015 showed increased uptake in the sternum, and metastatic relapse was confirmed on positron emission tomography/computed tomography (CT) imaging. The patient was treated with stereotactic ablative body radiotherapy and 9 cycles of capecitabine, followed by tamoxifen and goserelin.
In October 2016, new lesions were detected in the liver and bone. The patient underwent radiofrequency ablation procedures for her liver lesions and started systemic therapy. Over the next 5 years, she was treated with multiple systemic therapy regimens, including gemcitabine plus paclitaxel, docetaxel, palbociclib plus letrozole, everolimus plus exemestane, vinorelbine, fulvestrant, and weekly paclitaxel. However, at this time the metastatic lesions in the liver and bone were slowly progressed and new metastatic lesions were found in the lungs, pleura, and distant lymph nodes. While being treated with eighth-line therapy (eribulin), the patient agreed to undergo genomic profiling using plasma ctDNA to screen for any potential therapeutic target that had been missed, as there were no more viable standard therapy options. ctDNA profiling using AlphaLiquid100 (IMBdx) revealed a somatic mutation in BRCA1 (c.4336G>T, p.E1446*, COSMIC ID: COSV58803047) with a 6.68% allele frequency (Fig. 1A). Despite a lack of previous reports regarding PARPi responsiveness of this mutation, we recommended a therapeutic trial of olaparib based on the mutated locus being in the functional domain of BRCA1 (Fig. 2), as well as the Combined Annotation-Dependent Depletion (CADD) score being predictive of a deleterious mutation (C-score of 34) [7]. Additionally, the in-house homologous recombination repair deleteriousness (HRD) score (ranging from 0=benign mutation to 1=deleterious mutation; manuscript in preparation) calculated by IMBdx was 0.87, indicative of a high probability that this mutation was deleterious.
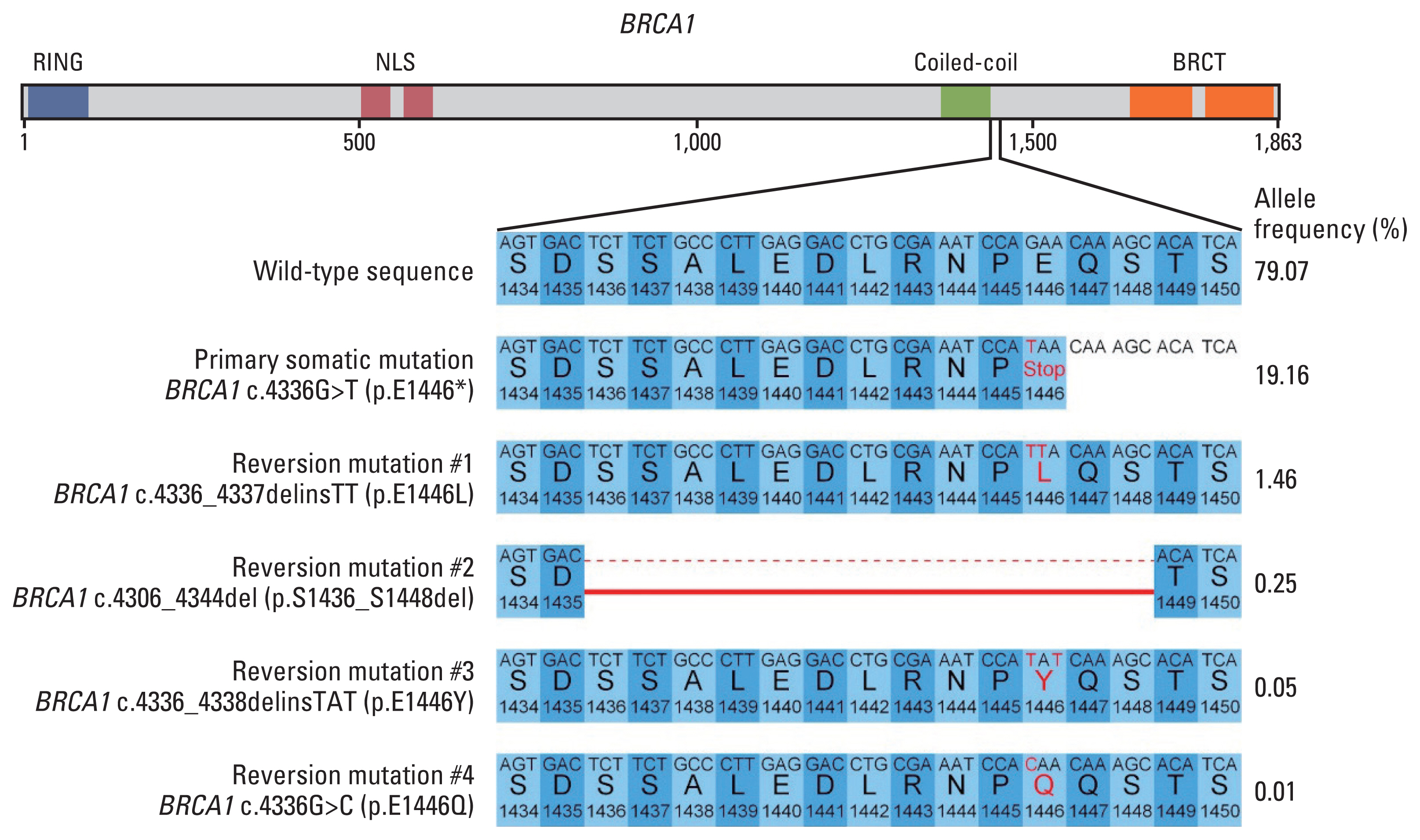
Primary and reversion BRCA1 mutations detected in this patient. (A) Functional domain annotation of mutations. (B) DNA and corresponding amino acid sequence changes of mutations, with allele frequencies, in April 2022. BRCT, BRCA1 C-terminal; NLS, nuclear localization signal.
Accordingly, the patient started off-label treatment with olaparib in August 2021, at a dose of 300 mg twice per day. The serum cancer antigen 15-3 started decreasing soon after initiating olaparib (Fig. 1A). Follow-up CT scans showed decreased size of multiple metastatic lesions in the liver and lungs (Fig. 1B and C), with the best response achieved in January 2022. At that time, there was a 55% decrease in the sum of the target lesions on CT, representing a partial response by Response Evaluation Criteria in Solid Tumor ver. 1.1. Follow-up liquid biopsies of ctDNA (AlphaLiquid100) were also performed and showed a decrease in the allele frequency of the previously detected somatic BRCA1 mutation to 0.25%.
After 8 months of olaparib, liver and chest CT scans obtained in April 2022 showed increases in the liver and lung lesions (194% increase from the best response), with the emergence of new liver lesions and a left pleural effusion (Fig. 1D). Olaparib treatment was thus discontinued, and ctDNA analysis at the time of discontinuation showed an increase of the previously detected somatic BRCA1 mutation to an allele frequency of 19.16%. New somatic mutations in BRCA1 that restored the truncated parts in the initial somatic mutation were also detected (BRCA1 p.E1446L, p.E1446Y, p. S1436_S1448del, and p.E1446Q) (Fig. 2). The allele frequency of a somatic estrogen receptor 1 (ESR1) mutation (c.1607T>A, p.L536H, COSMIC ID: COSV52795259) was also increased, from 0.05% to 5.95% (Fig. 1A). The last follow-up ctDNA analysis in July 2022 showed the presence of primary somatic BRCA1 mutation and two reversion mutations (BRCA1 p.E1446L and p.E1446Q) (S1 Table).
Discussion
In this report, we described a patient with metastatic breast cancer with a rare somatic BRCA1 mutation detected by ultra-sensitive ctDNA analysis of blood and who benefited from PARPi therapy, until the patient developed clinical resistance with BRCA1 reversion mutations.
BRCA1/2 mutated tumors are sensitive to PARPi because they lack the ability to repair DNA damage via the homologous recombination repair pathway. Approximately 10% of patients with breast cancer harbor germline mutations in BRCA1/2, and the clinical benefits of PARPi in these patients have been established in large clinical trials [1,2]. These agents have therefore been approved for use in breast cancer patients with germline BRCA mutations. In contrast, patient data are limited regarding somatic mutations in BRCA genes, but the known mechanisms of PARPi and emerging clinical data support the clinical utility of PARPi in these patients as well [3].
In addition, the BRCA1 E1446* mutation detected in this patient is a rare mutation reported only once in ovarian cancer in the COSMIC database, and there has been no prior report regarding PARPi responsiveness of breast cancer containing this mutation, which is classified as a tier III variant of unknown significance. However, the bioinformatic prediction of CADD and our in-house HRD score suggested the tumor’s BRCAness; we, therefore, prescribed off-label olaparib. The tumor responded quickly to this treatment, supporting the validity of our hypothesis.
This case illustrates the advantages of liquid biopsy. The patient required molecular profiling of her tumor to search for any available targets when disease progression was confirmed after treatment with most available chemotherapy regimens. However, the primary tumor was surgically resected 15 years ago, making it difficult to use. Moreover, there was a possibility that her resected primary tumor may not have been representative of her dominant cancer clone at the time of resistance, as the patient has been receiving various treatments for 15 years. Biopsies of metastatic lesions in the liver or sternum could have been considered to discover any druggable targets, but the patient’s general condition was poor, and biopsies at these sites are associated with a significant risk of complications. In this situation, non-invasive liquid biopsy using peripheral blood enabled elucidation of the molecular profile of the current tumor with minimal risk and identified its BRCAness.
Liquid biopsies of ctDNA also allowed serial analyses for monitoring temporal changes in mutation profiles (Fig. 1A). The allele frequency of BRCA1 E1446* was initially suppressed by olaparib but increased rapidly in concert with disease progression in April 2022. Interestingly, additional somatic mutations in BRCA1 were detected at the same or nearby genomic locations as the original somatic mutation. BRCA1 reversion mutations have been proposed as a mechanism of acquired PARPi resistance in ovarian and other cancers [5,6]. These reversion mutations restored a truncated portion of the functional domain in BRCA1 (Fig. 2), which reduced olaparib-derived synthetic lethality in the cancer and induced olaparib resistance. A concurrent ESR1 E536H mutation was also detected since January 2022, increasing from an allele frequency of 0.05% to 5.97%. This mutation in the ligand binding domain is known to result in a constitutively active form of estrogen receptor and resistance to aromatase inhibitors [8].
In conclusion, we reported a case of a patient with metastatic breast cancer who had a rare somatic BRCA mutation that responded to olaparib. Recent advances in technology including liquid biopsy and computational prediction of pathogenicity have enhanced the accessibility of genomic profiling in patients with advanced cancer and enabled the discovery of treatable targets in patients with otherwise no therapeutic options. The accumulation of reports of drug responsiveness in rare mutations expands our knowledge and will benefit future patients with refractory cancer.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
Written informed consent was obtained from the patient to publish this case report, and the protocol was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB number: H-1805-049-944), in accordance with the Declaration of Helsinki regarding biomedical research involving human subjects.
Author Contributions
Conceived and designed the analysis: Yoon JK, Lim Y, Kim TY.
Collected the data: Kim S, Lim Y, Kim TY.
Contributed data or analysis tools: Yoon JK, Kim HP, Kang JK.
Performed the analysis: Yoon JK, Ahn J, Kim HP, Kang JK.
Wrote the paper: Yoon JK, Ahn J, Bang D, Lim Y, Kim TY.
Conflicts of Interest
The authors declare no competing non-financial interests. The authors report the following potential financial competing interests: Yoon JK is a consultant for IMBdx; Kim HP, Kang JK, and Lim Y are employees of IMBdx; Bang D owns stocks in IMBdx and Celemics; Bang D received research funds and honoraria from IMBdx; Kim TY is a founder of IMBdx; and Kim HP and Kim TY are inventors on the patent application (No. 10-2022-0038856, filed on 29 Mar 2022).
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C1277). This research was also supported by IMBdx.
