Trastuzumab Combined with Irinotecan in Patients with HER2-Positive Metastatic Colorectal Cancer: A Phase II Single-Arm Study and Exploratory Biomarker Analysis
Article information
Abstract
Purpose
The human epidermal growth factor receptor 2 (HER2) is an established therapeutic target for various kinds of solid tumors. HER2 amplification occurs in approximately 1% to 6% of colorectal cancer. In this study, we aimed to assess the efficacy and safety of trastuzumab in combination with chemotherapy in HER2-positive metastatic colorectal cancer (mCRC).
Materials and Methods
An open-label, phase II trial (Clinicaltrials.gov: NCT03185988) was designed to evaluate the antitumor activity of trastuzumab and chemotherapy in HER2-positive digestive cancers excluding gastric cancer in 2017. Patients from this trial with HER2-positive, KRAS/BRAF wild-type, unresectable mCRC were analyzed in this manuscript. Eligible patients were treated with trastuzumab (8 mg/kg loading dose and then 6 mg/kg every 3 weeks) and irinotecan (120 mg/m2 days 1 and 8 every 3 weeks). The primary endpoint was the objective response rate.
Results
Twenty-one HER2-positive mCRC patients were enrolled in this study. Seven patients (33.3%) achieved an objective response, and 11 patients (52.4%) had stable disease as their best response. The median progression-free survival (PFS) was 4.3 months (95% confidence interval, 2.7 to 5.9). Four of the 21 patients (19.0%) had grade 3 adverse events, including leukopenia, neutropenia, urinary tract infection, and diarrhea. No treatment-related death was reported. Exploratory analyses revealed that high tumor tissue HER2 copy number was associated with better therapeutic response and PFS. Alterations in the mitogen-activated protein kinase pathway, HER2 gene, phosphoinositide 3-kinase/AKT pathway, and cell cycle control genes were potential drivers of trastuzumab resistance in mCRC.
Conclusion
Trastuzumab combined with chemotherapy is a potentially effective and well-tolerated therapeutic regimen in mCRC with a high HER2 copy number.
Introduction
The human epidermal growth factor receptor 2 (HER2), as a member of the epidermal growth factor receptor (EGFR) family, is a transmembrane glycoprotein receptor that could activate several signal transduction pathways, including the mitogen-activated protein kinases (MAPK) pathway and the phosphoinositide 3-kinase (PI3K)/AKT pathway, and regulate cell proliferation and apoptosis [1]. HER2 overexpression has been characterized as a valuable prognostic biomarker and therapeutic target in the treatment of breast and gastric cancers [2,3]. Trastuzumab combined with chemotherapy was approved to be effective and was recommended as the first-line standard therapy for HER2-positive gastric and breast cancers [4,5].
The frequency of HER2 overexpression or amplification in colorectal cancer (CRC) ranges between 1% to 6%, which is lower than that of breast and gastric cancers [6–9]. The rarity of HER2 positivity and absence of a recognized criterion in CRC impeded the development of HER2-targeted clinical trials in the early days. Although the influence of HER2 positivity on the prognosis of CRC remains controversial, both preclinical and clinical evidence have found an association between HER2 positivity and resistance to anti-EGFR antibodies in metastatic colorectal cancer (mCRC), resulting in an unmet therapeutic need for this particular population [10,11].
Two early clinical trials evaluated the efficacy of HER2-targeted therapy and chemotherapy in CRC. Clark et al. [12] tested folinic acid, fluorouracil, and oxaliplatin (FOLFOX) in combination with trastuzumab regimen in pretreated HER2+/3+ mCRC and achieved an objective response rate of 24%. In 2004, Ramanathan et al. [13] performed a phase II trial evaluating the efficacy of trastuzumab in combination with irinotecan in HER2 2+/3+ mCRC. Of the seven patients enrolled in this study, five had a partial response. This study was prematurely closed because of low accrual, leaving the question of the efficacy of chemotherapy and trastuzumab in HER2-positive mCRC unanswered. However, the initial results suggested that the therapeutic effect of this regimen was worth further investigation.
In 2017, we initiated an open-label, phase II basket trial to evaluate the clinical activity and safety profiles of trastuzumab in combination with chemotherapy in HER2-positive pretreated metastatic digestive system tumors excluding gastric cancer. Here, we present the treatment outcomes of HER2-positive mCRC in this trial. And concomitant molecular biomarker detection was conducted to investigate the predictive biomarkers and resistant mechanisms.
Materials and Methods
1. Study design and participants
The study utilized an open-label, multicenter, basket phase II trial (NCT03185988) design. Patients with HER2-positive metastatic digestive system tumors, including biliary tract cancer (BTC) (Arm1); esophageal squamous cell carcinoma (ESCC) (Arm2), digestive system tumors excluding BTC, ESCC, gastric cancer and CRC (Arm3), and CRC (Arm4) were screened for treatment. Arm1, 2, and 3 were all closed due to inadequate recruitment. Thus, only CRC patients enrolled in Arm4 are described in this manuscript. Patients in Arm4 had histologically and radiologically confirmed mCRC. HER2 positivity was defined as either a HER2 expression score of +3 by immunohistochemistry or HER2 expression score of +2 with a HER2:centromeric region of chromosome 17 (CEP17) ratio above 2.0 by fluorescence in situ hybridization (FISH) test in more than 10% of the tumor cells. Sanger sequencing was performed to confirm the KRAS and BRAF status of each patient. All eligible patients had to have wild-type KRAS, and BRAF genes. Patients were required to have a metastatic disease with at least one measurable lesion according to the response criteria evaluation in solid tumors (RECIST) ver. 1.1. Other key inclusion criteria included: age between 18 and 75; an Eastern Cooperative Oncology Group performance status of 0 or 1, progression after at least one standard systemic therapy, adequate bone marrow, hepatic and renal function. Detailed inclusion and exclusion criteria are available in the S1 Table. Using the Minimax Simon’s two-stage design, we planned to accrue 25 HER2-positive mCRC to differentiate between objective response rate (ORR) of 10% and 30% with type I error of 5% and power of 80% (one-tailed test). However, the CRC cohort was terminated prematurely in January 2021 due to slow enrollment, and 21 patients were enrolled.
2. Treatment procedures
Chemotherapy and trastuzumab were given every 3 weeks for the first six cycles and then trastuzumab was given as the maintenance treatment to reduce the adverse effect of chemotherapy. Considering most patients had been exposed to oxaliplatin in the first-line treatment, irinotecan was chosen as the chemo in this trial. Irinotecan was administered intravenously using a dose of 120 mg/m2 on day 1 and day 8 of each cycle. Trastuzumab was given intravenously every 3 weeks until disease progression, withdrawal of consent, intolerable toxicity, or death. During treatment, trastuzumab was given at a dose of 8 mg/kg in the first cycle and followed by 6 mg/kg every 3 weeks. The first infusion was administered over 90 minutes, and subsequent infusions are to be given over 30 minutes if the first infusion was well tolerated.
3. Treatment response assessment and adverse events
Contrast-enhanced computed tomography scan was performed every 6 weeks to assess treatment response. Tumor response was evaluated by investigators using the RECIST 1.1 criteria. Adverse events were evaluated continuously at every visit by investigators according to the Common Terminology Criteria for Adverse Events ver. 4.03. Laboratory tests, including complete routine full blood count, biochemical blood testing, urine analysis, and plasma tumor markers, were performed on the first day of each cycle. In addition, an echocardiogram was performed before treatment and every 3 months during treatment to monitor the left ventricular ejection fraction.
4. Outcomes
The primary endpoint for this study was the radiographic response rate which was defined as the percentage of patients who achieved a complete or partial response. The secondary endpoints included disease control rate (defined as the proportion of patients with an objective response or stable disease as the best response), progression-free survival (PFS) (defined as the time from first treatment administration to disease progression or death), overall survival (OS) (defined as the time from first treatment administration to death or last follow-up), duration of response (DOR) (defined as the time from first response to disease progression or death), and treatment-related adverse events (TRAEs).
5. Sample collection and genetic testing
Genetic testing was conducted using the tumor tissue and plasma samples to investigate the predictive biomarkers and potential resistance-related alterations. Tumor tissue was obtained at the first diagnosis of each patient. Blood samples were collected before treatment and after disease progression to analyze the acquired mutations in the circulating tumor DNA (ctDNA). Detailed sequencing information and the procedures of data processing are provided in the Supplementary Methods.
6. Statistical analysis
Descriptive statistics were used to summarize the demo-graphic characteristics and treatment outcomes of all patients involved. Survival data was analyzed using the Kaplan-Meier method. The log-rank test was used to compare the PFS and OS. All reported p-values were based on two-tailed testing, and a p-value below 0.05 was deemed statistically significant. All statistical analyses were performed using GraphPad Prism software 8.3.0 (GraphPad Software Inc., San Diego, CA) and R software ver. 4.1.
Results
1. Patient characteristics
A total of 21 HER2-positive mCRC patients were enrolled in this study. The demographics and clinical characteristics are summarized in Table 1. Seven patients were female, and the median age was 54.5 years (range, 30 to 67 years). Ten patients had rectal cancer, and 11 had left-side colon cancer. As for the metastatic location, 13 patients had metastatic lesions in more than one organ. Lung, live, lymph node, and bone metastasis was observed in 61.9%, 47.6%, 33.3%, and 9.5% of patients, respectively. The majority of the patients (13/21) had received one prior systemic treatment regimen, and six patients had received two lines of systemic therapy. The other two patients had received three lines of systemic therapy. Nine of them have been exposed to irinotecan in previous treatment. In addition, eight patients had been treated with EGFR-targeted antibodies. HER2 testing was performed using the tumor tissues derived from the primary tumor in 19 patients and metastatic lesions in two patients. Sixteen of these patients were HER2 3+, and the remaining were HER2 2+ with the HER2:CEP17 ratio ranging from 3.8 to 5.8. All patients were confirmed to be KRAS/BRAF wild-type by Sanger sequencing and no microsatellite instable high CRC was included.
2. Antitumor activity
Until February 2022, the data collection cutoff point, only two patients remained in the study. The other 19 patients had discontinued the treatment because of disease progression. All patients were eligible for treatment efficacy evaluation, and the median follow-up time was 14.0 months. Fig. 1 illustrates the largest percent change in the target lesion size for each patient. Tumor shrinkage of any extent was observed in 16 out of 21 patients. Seven patients achieved an objective response during treatment, and the objective response rate was 33.3%. All responders were HER2 3+ on the immunohistochemistry test and their HER2 copy number by the FISH test was comparable with non-responders (p=0.585). Stable disease was observed in 11 patients, including five lasting for four months or longer. Overall, disease control was achieved in 18 patients; the disease control rate was 85.7%.
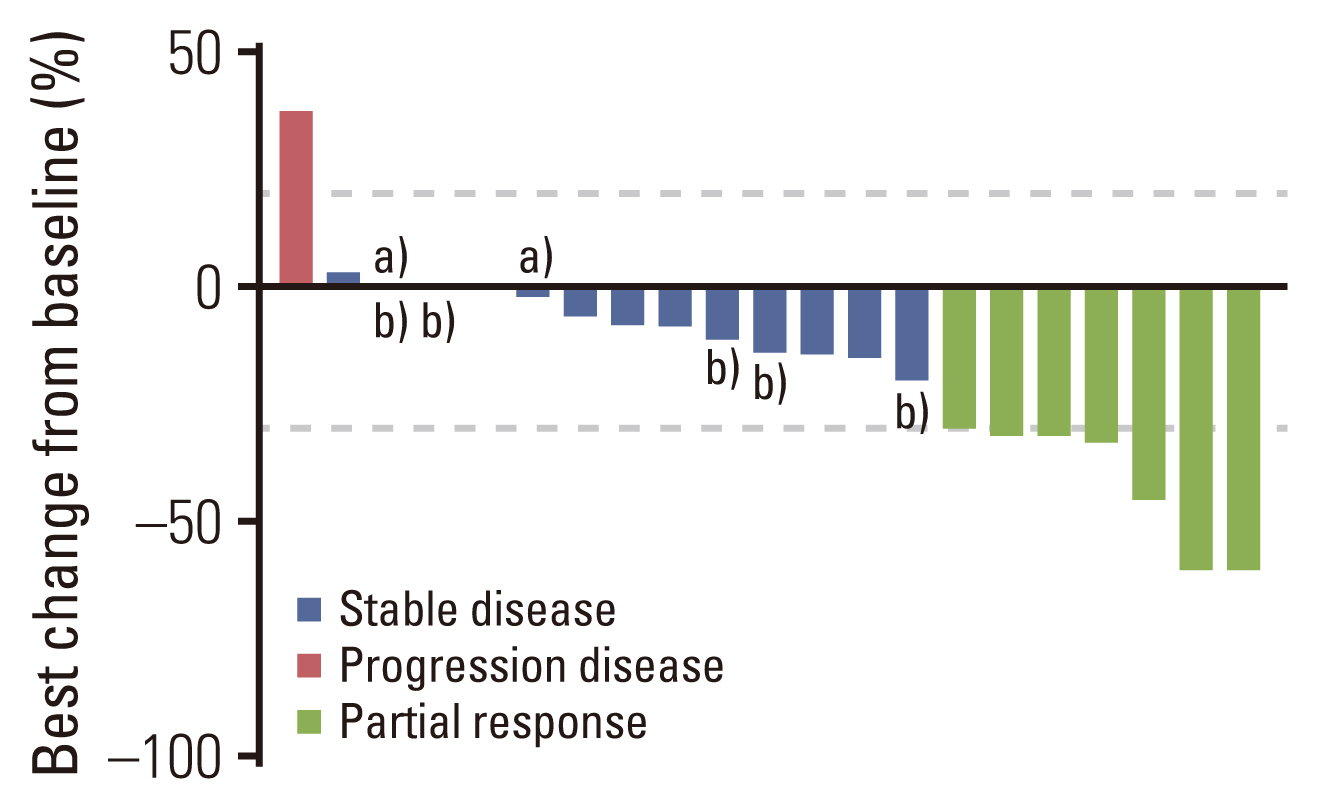
Best change from baseline in target lesion size by each patient. FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2. a)Progression of non-target lesions or ccurence of new lesions, b)HER2 2+FISH positive.
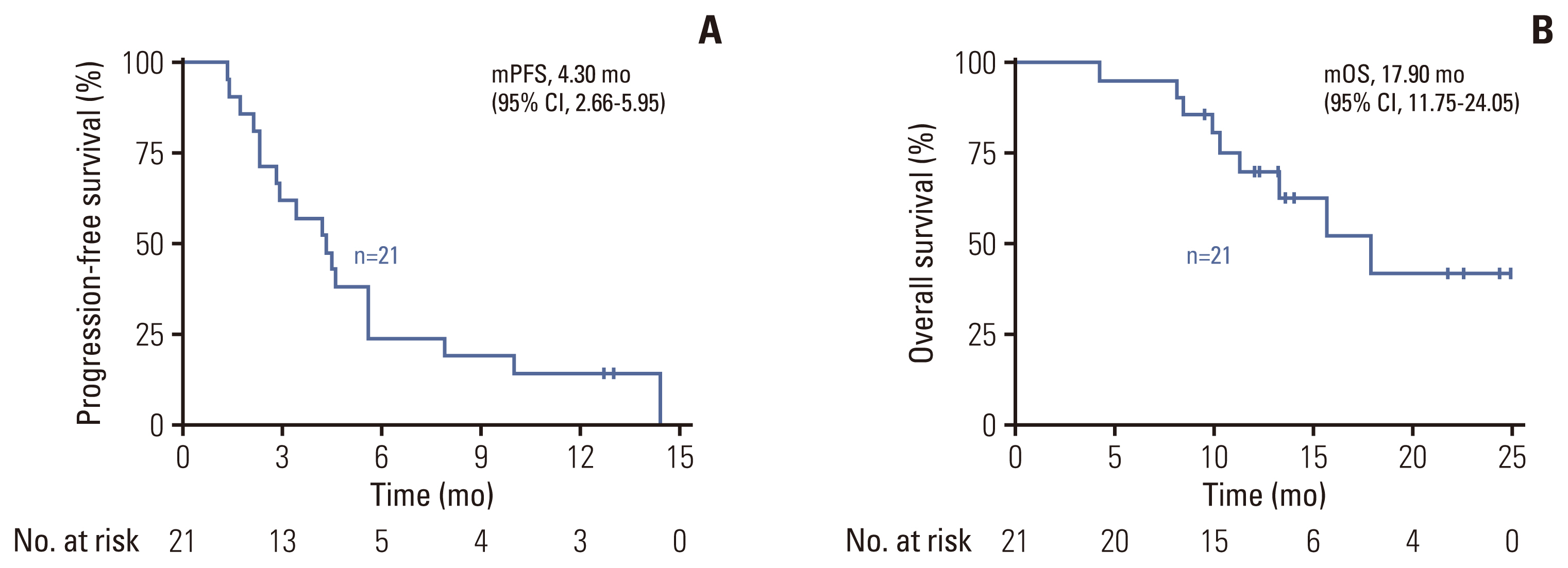
The median PFS of the entire cohort was 4.3 months (95% confidence interval [CI], 2.7 to 5.9), with two of them having ongoing responses by the cutoff date (Fig. 2A). Nine patients died due to tumor progression, and the unmatured median OS was 17.9 months (95% CI, 11.8 to 24.1) (Fig. 2B). Out of the 16 HER2 3+ patients, the median PFS was 4.5 months (95% CI, 2.7 to 5.9) which was better than that of HER2 2+ patients with a median PFS of 2.9 months (95% CI, 1.2 to 4.6), though statistical significance was not reached (p=0.094). Five of 21 patients remained on disease control at 6 months after treatment initiation. Amongst the eight patients with prior EGFR antibody therapy, objective response was achieved in only one of them, and the median PFS was 2.3 months.
3. Treatment-related adverse events
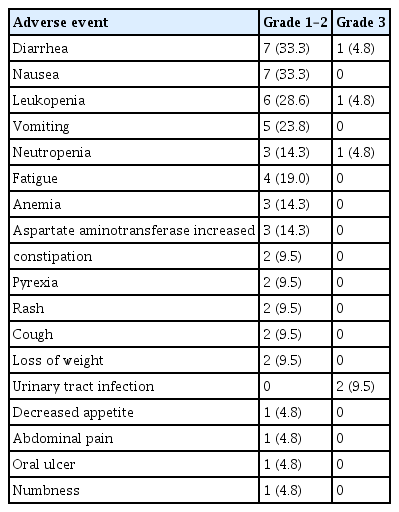
The grading of adverse events for all patients is listed in Table 2. TRAEs were reported in 85.7% (18/21) of patients. The most common TRAEs were diarrhea (8/21), nausea (7/21), leukopenia (7/21), vomiting (5/21), neutropenia (4/21), and fatigue (4/21). Most TRAEs were classified as grade 1 or 2, as expected. Four patients experienced grade 3 TRAEs: one patient had leukopenia and neutropenia, two had urinary tract infections, and one had diarrhea. None of the patients terminated treatment due to unacceptable toxicity, and no treatment-related death was observed. No ejection fraction decrease was observed on echocardiography.
4. Genetic drivers of resistance
To determine the potential genetic drivers of resistance in mCRC, we analyzed the genetic profiles of pretreatment tumor tissues (n=20), pretreatment (n=18), and after-progression plasma samples (n=17) based on a customized panel of 733 cancer-related genes. The mutational characteristics of baseline tissue and ctDNA exhibited prominent consistency, with HER2, TP53, APC, RARA, TOP2A, and SMAD4 being identified as the most frequently mutated genes (S2A and S2B Fig.). The previously reported mutations known to be involved in resistance to HER2-targeted therapy, including mutations in KRAS, NRAS, BRAF, PIK3CA, and MYC genes, as well as emergent mutations identified in our study after exposure to treatment progression are summarized in Fig. 3.
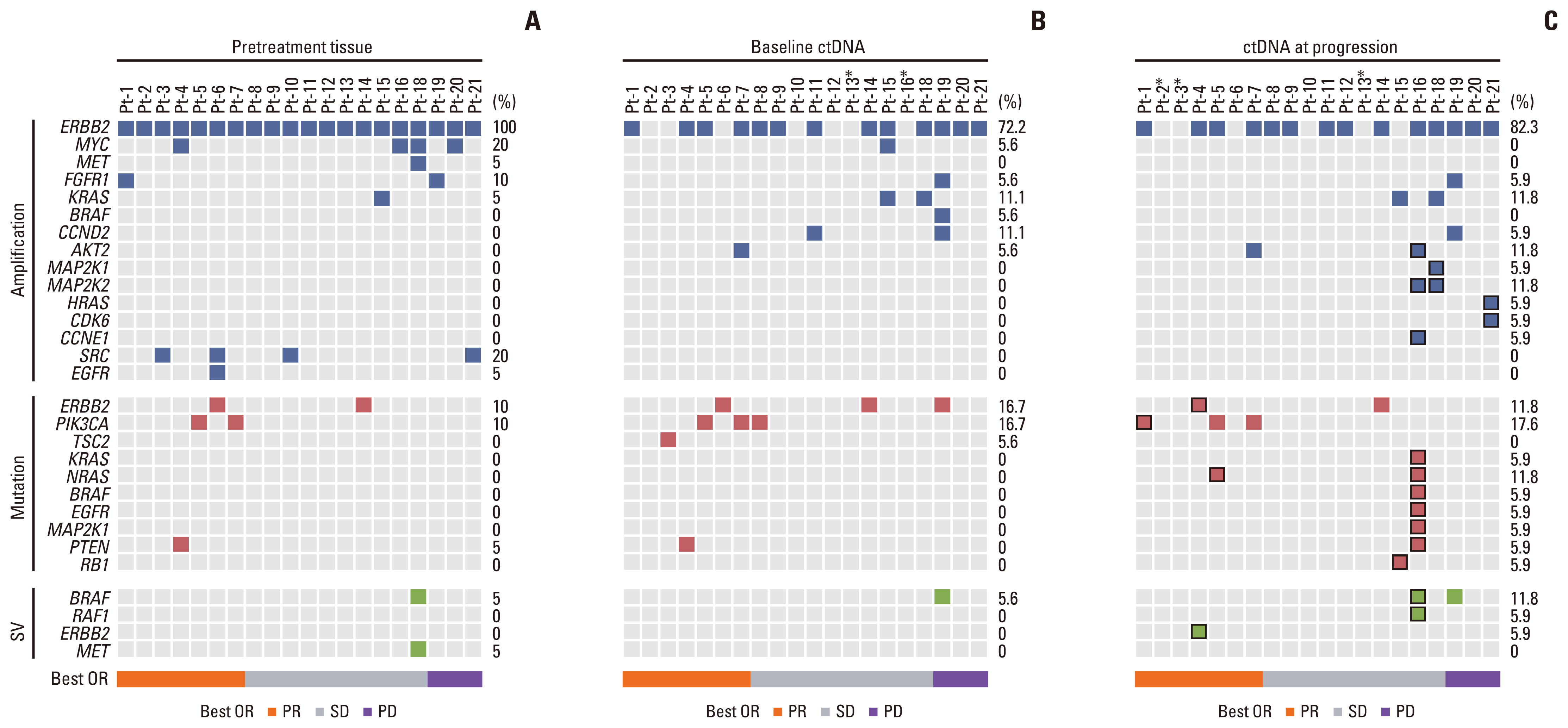
Molecular alterations in trastuzumab resistance-related genes at baseline and acquired mutations after trastuzumab treatment. (A) Pretreatment tissue. (B) Baseline circulating tumor DNA (ctDNA). (C) ctDNA at progression, the acquired mutations were marked with a black block. Asterisks indicate no available samples for analysis in this patient. Best OR, best of response; PD, progression disease; PR, partial response; SD, stable disease; SV, structural variation.
Since heterogeneity of HER2 expression was a critical mechanism of HER2 resistance in gastric cancer, we analyzed the HER2 expression and copy number before treatment and after disease progression (S3 Table). HER2 amplification was detected in 20 out of 20 (100.0%) of the baseline tissues, 13 out of 18 baseline plasma samples, and 15 out of 17 plasma samples after disease progression. Patients with a high tumor tissue HER2 copy number (copy number > 26) had a better response to trastuzumab plus chemotherapy with a median PFS of 5.6 months compared to the low copy number group (copy number ≤ 26) with a median PFS of 2.3 months (p=0.004) (Fig. 4A). Loss of ctDNA HER2 amplification was only observed in patient No. 15. The immunohistochemical test in this patient confirmed a transition from HER2 2+ to HER2 1+, suggesting that the HER2 amplified clone might have been eliminated by the HER2-targeted therapy (Fig. 4B).

Genetic characteristics associated with trastuzumab resistance. (A) Progression-free survival by human epidermal growth factor receptor 2 (HER2) gene copy number variation; HER2 amplification copy number ranking the top two-thirds were defined as high copy number variation (CNV), and the others were defined as low CNV (high ERBB2 CNV > 26; low ERBB2 CNV ≤ 26). (B) HER2 expression of patient No. 15 before treatment and after progression on trastuzumab treatment. (C) Progression-free survival of circulating tumor DNA mitogen-activated protein kinase (MAPK) pathway wild-type (WT; n=15) and mutant (MUT; n=3, 2 with KRAS amplification and one with BRAF amplification) HER2-positive metastatic colorectal cancer. (D) Multiple acquired mutations in HER2 gene in patient No. 16 after exposure to trastuzumab.
Alterations of the MAPK pathway were the most common mechanisms associated with the anti-EGFR antibody resistance in CRC. Thus, we analyzed the MAPK pathway mutations in detail. Although Sanger sequencing was performed to confirm the wild-type KRAS, and BRAF before enrollment, KRAS and BRAF alterations were identified in three patients at baseline ctDNA detection including two with KRAS amplification and one with BRAF amplification and fusion. Baseline tumor tissue genotyping showed that KRAS or BRAF amplification was not detected in pretreatment tumor samples in two of the three patients. In these two cases, the KRAS and BRAF amplification could be acquired after treatment with anti-EGFR antibody. None of the three patients responded to trastuzumab combined with chemotherapy, and the median PFS was significantly shorter than those without MAPK pathway mutation at baseline (5.6 vs. 2.1 months, p=0.008) (Fig. 4C). Besides, emergent alterations in the MAPK pathway were acquired in four patients after disease progression. In patient number 16, abundant MAPK pathway alterations were acquired at progression disease, including KRAS, NRAS, BRAF, EGFR, MEK1 activating mutations, BRAF fusion, and MEK2 amplification, while NRAS mutation, HRAS amplification, and MEK2 amplification were identified in the other three patients (Fig. 3C). These results indicate that MAPK pathway mutations including non-canonical mutation of RAS and BRAF genes have an important role in resistance to trastuzumab in CRC.
Patient No. 4 had a partial response during trastuzumab treatment with a PFS of 10.0 months. In this patient, HER2 amplification was maintained after radiographic progression, and multiple acquired HER2 mutations were detected, including kinase domain mutations (D769Y, D769H, V777L, and I767M), a juxtamembrane domain mutation (S653C), and a splicing variant of the HER2 lacking exon-16 (HER2 Δ16) (Fig. 4D). Preclinical evidence showed that tyrosine kinase domain mutants of HER2, like D769Y/H, V777L, could enhance the activity of the HER2 signaling pathway and induce resistance to trastuzumab [14,15]. Furthermore, HER2 Δ16 could form a stable HER2 dimer in an SRC-dependent manner and drive resistance to trastuzumab in breast cancer cell lines [16,17].
Furthermore, three out of 17 patients developed mutations in the PI3K pathway after disease progression, of which one patient acquired a PIK3CA C420R mutation, one patient acquired AKT2 amplification, and another patient acquired AKT2 amplification with a concomitant PTEN F154L mutation. Furthermore, acquired genetic alterations in genes related to cell cycle control were found in four patients, but the significance of these mutations remains uncertain. Overall, multiple putative resistance-associated mutations were involved in the resistance of trastuzumab in HER2-positive CRC. These mutations were enriched in the MAPK pathway, PI3K/AKT pathway, cell cycle control-related genes, and the HER2 gene itself. Notably, the ctDNA sequencing suggested that polyclonal resistant alterations were frequent in HER2-amplified CRC, indicating heterogeneity in the development of resistance to systemic therapy.
Discussion
This phase II study suggests that the irinotecan and trastuzumab combination is an effective regimen in pretreated HER2-positive mCRC. In our cohort, the objective response was achieved in 7/21 (33.3%) of patients with a median DOR of 5.6 months. And the safety profile was acceptable, with most TRAEs being grade 1 or 2.
Generally, the response rate of the second-line irinotecan-based chemotherapy was 10% to 20% in mCRC in previous studies [18,19]. And the ORR declined to less than 5% in the context of standard third-line regorafenib or fruquintinib [20,21]. In our study, the ORR of the irinotecan and trastuzumab combination was 33.3% which provided a promising strategy for HER2-positive pretreated mCRC considering nearly half of the participants had received at least two lines of systemic treatment. Furthermore, the post hoc analysis of tumor tissue genomic profiles of HER2-positive mCRC showed that superior clinical benefit was obtained in patients with a high HER2 copy number. The efficacy of HER2-targeted therapy in mCRC has been evaluated in several other clinical trials. The Mypathway study was a phase 2a basket study evaluating pertuzumab and trastuzumab in HER2 amplified tumors. Among the 43 KRAS wild-type mCRC patients involved in the trial, the objective response rate was 40%, with a median PFS of 5.3 months [22]. Similarly, the trastuzumab plus lapatinib combination in the HERACLES trial achieved an objective response rate of 30%, and the median PFS was 5.2 months [23]. It should be noted that patients enrolled in this study were less heavily pretreated compared with Mypathway and HERACLES trials. Nevertheless, the response rate of trastuzumab and irinotecan was superior than that of chemotherapy alone in the second-line treatment of mCRC. Our results support trastuzumab and irinotecan combination could be a potential treatment option for HER2-positive mCRC, especially for those with a high HER2 copy number.
With the increasing use of HER2-targeted therapy in CRC, there is a need to understand the molecular drivers of trastuzumab resistance in CRC to monitor disease progression and overcome resistance in the additional lines of treatment. Loss of HER2 positivity was reported in about 30% of gastric cancer patients that progressed on trastuzumab, especially in HER2 2+ tumors [24]. Following our ctDNA analysis, a change from a HER2-amplified tumor into HER2-negative after treatment was only noted in one out of the 13 patients. No significant decrease in ctDNA HER2 copy number was observed. Our data suggested that though less commonly seen, loss of HER2 positivity is a potential resistance mechanism in CRC treated with HER2-targeted agents, thus the HER2 expression status should be re-assessed before subsequent anti-HER2 therapy.
The Mypathway study showed that in HER2-amplified CRC patients with KRAS mutations, the overall response rate was only 8%, versus 40% in KRAS wild-type patients [25]. In a post hoc analysis of ctDNA genetic profiles of patients enrolled in the HERACLES trial, RAS or BRAF mutations were observed in six out of the seven refractory patients, but only three of 22 patients with clinical benefit, which further confirmed the involvement of MAPK activation in HER2 blockade resistance [26]. Given these findings, patients with hot spot mutations in KRAS/BRAF genes were excluded. However, in the baseline ctDNA analysis, two patients with KRAS amplification and one with BRAF amplification were identified. None of them benefit from trastuzumab treatment. Notably, in two of them, the amplification was observed in baseline plasma samples but not in treatment-naive tumor tissues. The potential explanation was that previous EGFR antibody exposure might have selected the resistant KRAS/BRAF amplified subclones. We also identified acquired mutations in the KRAS, HRAS, NRAS, BRAF, and MAP2K1 genes in ctDNA following disease progression in four patients. Our study presents the significance of MAPK pathway activation in primary and secondary resistance to HER2-targeted therapy in CRC. KRAS and BRAF amplification, either original or acquired after anti-EGFR treatment, could also drive the primary resistance to trastuzumab in mCRC.
Additionally, our study identified acquired mutations in the PI3K/AKT pathways, cell cycle-related genes, and HER2 gene after disease progression. In breast cancer, the aberrant activation of the PI3K/AKT pathway caused by gain-of-function mutations in the PIK3CA, AKT1, and PIK3R1 genes, or loss of function in the PTEN gene were the most common genetic events, occurring in more than 30% of cases, involved in HER2 inhibitors resistance [27]. Mutations on the target HER2 gene, either impeding the drug binding or activating the kinase, have also been identified in HER2-resistant gastric and breast cancer [28]. Furthermore, deregulation of the cell cycle control genes, such as cyclin E and CDK2, was also associated with HER2 inhibition resistance in breast cancer [29]. Generally, our study suggested that the molecular drivers of resistance to HER2-targeted therapy in mCRC are similar to those observed in gastric and breast cancers. However, at the same time, differences were also noted, as exemplified by the remarkable involvement of the MAPK pathway mutations and relatively rare loss of HER2-positivity.
This study has some limitations that need to be acknowledged. The trial was based on a single-arm designation and a relatively small sample size. The low number of patients with different molecular characteristics limited the interpretability of predictive biomarker analysis. Although it was initially designed as a multicenter study, only one center provided evaluable data ultimately, potentially leading to selection bias. In addition, the acquired resistance mechanism of HER2-targeted therapy was only based on the ctDNA but not re-biopsy tumor tissue. As a result, resistant tumor subclones might have been missed due to low tumor DNA shedding.
Trastuzumab combined with chemotherapy is a well-tolerated and potentially effective therapeutic regimen in the management of HER2-positive mCRC. The use of comprehensive tumor tissue and ctDNA genotyping is recommended to identify the patients that are most likely to benefit from HER2-targeted therapy. Furthermore, the diverse acquired resistance mechanisms observed in our study could be used to determine a personalized therapy after the failure of HER2-targeted therapy.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. The study (approval number: 2017YJZ17) protocol was reviewed and approved by the ethics committee of Peking University Cancer Hospital and other clinical centers involved in this trial. Written informed consent was obtained from all patients participating in the study.
Author Contributions
Conceived and designed the analysis: Wang X, Li J, Shen L.
Collected the data: Xu T, Wang Z.
Contributed data or analysis tools: Xu T, Xin Y, Wang Z, Gong J, Zhang X, Li Y, Ji C, Sun Y, Zhao F, Huang D, Bai Y.
Performed the analysis: Xu T, Wang X.
Wrote the paper: Xu T, Wang X, Li J, Shen L.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
We gratefully thank all the patients for their participation in this clinical trial and all the stalls for their work and commitment to the study. The authors thank for 3D Medicine providing assistance in genotyping and analysis. We would like to thank TopEdit (www.topeditsci.com) for the English language editing of this manuscript. This project is supported Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (code: ZYLX202116).