A Phase II Trial of S-1 and Oxaliplatin in Patients with Metastatic Breast Cancer Previously Treated with Anthracycline and Taxane (KCSG-BR07-03)
Article information
Abstract
Purpose
This single-arm phase II trial investigate the efficacy and safety of S-1 plus oxaliplatin (SOX) in patients with metastatic breast cancer.
Materials and Methods
Patients with metastatic breast cancer previously treated with anthracyclines and taxanes were enrolled. Patients received S-1 (40–60 mg depending on patient’s body surface area, twice a day, day 1–14) and oxaliplatin (130 mg/m2, day 1) in 3 weeks cycle until disease progression or unacceptable toxicity. The primary endpoint was objective response rate (ORR) according to Response Evaluation Criteria in Solid Tumor 1.1. Secondary endpoints included time-to-progression (TTP), duration-of-response (DoR), overall survival (OS), and adverse events.
Results
A total of 87 patients were enrolled from 11 institutions in Korea. Hormone receptor was positive in 54 (62.1%) patients and six (6.9%) had human epidermal growth factor receptor 2–positive disease. Forty-eight patients (85.1%) had visceral metastasis and 74 (55.2%) had more than three sites of metastases. The ORR of SOX regimen was 38.5% (95% confidence interval [CI], 26.9 to 50.0) with a median TTP of 6.0 months (95% CI, 5.1 to 6.9). Median DoR and OS were 10.3 months (95% CI, 5.5 to 15.1) and 19.4 (95% CI, not estimated) months, respectively. Grade 3 or 4 neutropenia was reported in 28 patients (32.1%) and thrombocytopenia was observed in 23 patients (26.6%).
Conclusion
This phase II study showed that SOX regimen is a reasonable option in metastatic breast cancer previously treated with anthracyclines and taxanes.
Introduction
With the advent of novel therapeutic agents and earlier cancer screening, the survival of breast cancer continues to improve and the 5-year survival rate of breast cancer exceeds 90% [1]. However, the 5-year survival rate of stage IV disease remains poor (27%) [1]. Treatment options for metastatic breast cancer (MBC) include cytotoxic chemotherapy, endocrine therapy, human epidermal growth factor receptor 2 (HER2)–directed therapy, and recently approved immune checkpoint inhibitors, depending on tumor subtype and biomarkers. Cytotoxic chemotherapy is the main backbone treatment of HER2-positive or triple-negative disease. In addition, it is the treatment of choice for endocrine therapy resistant hormone receptor (HR)–positive breast cancer. Traditionally, anthracyclines and taxanes are the preferred chemotherapy agents in MBC. However, patients will substantially develop resistance or intolerance to these agents. Various single agents or combination regimens including eribulin, capecitabine, gemcitabine, vinorelbine, platinum, and ixabepilone are used in breast cancer patients refractory to anthracyclines and taxanes [2,3]. However, their clinical efficacy as a single agent remains modest and combination regimens show an exacerbated toxicity profile which limits their routine use. Therefore, development of effective and tolerable combination regimen for the management of refractory MBC is needed.
S-1 is an oral fluoropyrimidine agent that consists of tegafur (a prodrug of 5-fluorouracil [5-FU]) and 2 modulators (gimeracil and oteracil) at the molar ratio of 1:0.4:1. Gimeracil increases 5-FU concentration in the plasma and tumor tissues by inhibiting degradation of 5-FU. Orally administered oteracil selectively distributed in the gastrointestinal tract and suppresses gastrointestinal toxicity of 5-FU by inhibiting phosphorylation of 5-FU [4]. S-1 has shown clinical efficacy in first-line or second-line treatment of MBC with an objective response rate (ORR) of 41.7% [5]. In a randomized phase III trial, S-1 was non-inferior to taxane or anthracycline-containing regimens in first-line treatment for MBC [6,7]. Moreover, S-1 showed an ORR of 21.8% as a salvage treatment in MBC [8]. Compared to another oral fluoropyrimidine agent, capecitabine, a multivariate analysis showed that S-1–based chemotherapy showed non-inferior antitumor efficacy and better safety profile (hand-foot syndrome) as first-line treatment for advanced gastric carcinoma, at least in Asian [9]. In addition, S-1 showed lower incidence of hand-foot syndrome compared with capecitabine, with comparable efficacy in Western patients with metastatic colorectal cancer [10].
Oxaliplatin is a platinum derivative containing an oxalate ligand, which was developed to overcome resistance to the first- and second-generation platinum complexes [11–13]. Oxaliplatin shows wide ranges of in vitro cytotoxic and in vivo antineoplastic activities which are different from that of cisplatin or carboplatin [13]. In MBC patients who were previously treated with anthracyclines and taxanes, single agent oxaliplatin was active with an ORR of 21% [14]. Oxaliplatin provides a synergistic antineoplastic effect when combined with 5-FU in several in vivo tumor models [12]. Provided by preclinical evidence of synergism of oxaliplatin and 5-FU, this combination regimen has been investigated and widely used in advanced solid cancers including colorectal cancer, gastric cancer, and breast cancer [15–18]. In anthracycline- and taxane-refractory MBC, the combination of oxaliplatin plus 5-FU was an active regimen with an ORR of 27%-34% and time to progression of 4.9 and 5.3 months [17,18]. S-1 has the advantage over 5-FU to have a more convenient route of administration (it avoids 48 hours infusion) and less gastrointestinal toxicity albeit equivalent efficacy.
Recently, 5-FU has been substituted by S-1 to treat colorectal cancer and stomach cancer based on a studies showing comparable efficacy and better safety profiles [19,20]. In a retrospective study performed in advance triple-negative breast cancer, S-1 and oxaliplatin (SOX) was an effective and tolerable combination regimen [21]. The purpose of this trial is to evaluate the clinical efficacy and toxicity profiles of SOX in MBC.
Materials and Methods
1. Study design and population
The KCSG-BR07-03 trial was an open-label, multicenter, single-arm, phase II trial conducted in 11 institutions in Korea. Patients with histologically confirmed MBC previously treated with anthracyclines and taxanes were eligible. Other main inclusion criteria included: age over 18 years; Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2; adequate bone marrow, hepatic, and renal function (hemoglobin ≥ 9.0 g/dL, absolute neutrophil count ≥ 1,500/μL; platelet count ≥ 100,000/μL, total bilirubin ≤ 1.5× upper limit of normal [ULN]; serum transaminases [aspartate aminotransferase (AST)/alanine transaminase (ALT)] ≤ 2.5× ULN; [AST/ALT ≤ 5.0× ULN was acceptable in patients with known hepatic metastases]; serum creatinine ≤ 1.5 mg/dL). Key exclusion criteria were as follows: pregnant or breast feeding women; prior treatment with S-1, capecitabine, or platinum; symptomatic brain metastases; patients with ongoing grade 3 or higher neurotoxicity; treated with 4 or more lines of chemotherapy for metastatic disease. HER2-positive patients could be enrolled if they were not candidates of HER2 targeted agents. Measurable disease defined by Response Evaluation Criteria in Solid Tumor (RECIST) 1.1 was mandatory [22].
The study protocol was reviewed and approved by the institutional review board of each participating center. Written informed consent was obtained from each patient before enrollment. This study was carried out in accordance with the recommendations of the Declaration of Helsinki for biomedical research involving human subjects and the Guidelines for Good Clinical Practice (ClinicalTrial.gov Identifier: NCT00527930).
2. Study procedure
Patients received oxaliplatin 130 mg/m2 as a 2-hour intravenous infusion on day 1 and oral S-1 determined by body surface area (< 1.25 m2, 80 mg; ≥ 1.25 to < 1.5 m2, 100 mg; ≥ 1.5 m2, 120 mg) administered twice daily on days 1 to 14 of a 21-day cycle. Treatment was continued until disease progression, unacceptable toxicity, or withdrawal of patient consent. Baseline assessments included medical history, full physical examination, electrocardiography, chest X-rays, abdominal and chest computed tomography (CT) scans, complete blood counts, serum electrolytes and chemistry, and urine analysis. Tumor response was assessed using RECIST criteria 1.1 by the local investigator at baseline and every two cycles (6 weeks) [22]. Toxicity was evaluated at each cycle per the National Cancer Institute Common Terminology Criteria for Adverse Events, ver. 3.0.
3. Statistical analysis
The primary endpoint of the study was ORR (complete response [CR]+partial response [PR]) with SOX according to RECIST 1.1. Secondary endpoints included time to progression (TTP), duration of objective response (DoR), overall survival (OS), and toxicities. TTP was calculated from the first day of drug administration to disease progression. Deaths without progressive disease were censored in the analysis of TTP. OS was measured from the first day of drug administration to the date of death from any cause. TTP and OS were estimated using the Kaplan-Meier method. Response rates were compared using chi-square test or Fisher exact test as appropriate. DoR was calculated as the time from first day of drug administration to progression in patients who had a best overall response of CR or PR. Analyses of treatment effects were adjusted for multiple covariates using a multivariate Cox proportional hazards model. Differences were considered statistically significant at p < 0.05 with a two-tailed test. Statistical analysis was performed with SPSS software for Windows ver. 20.0 (IBM Corp., Armonk, NY).
The expected response rate of SOX was 35% based on previous trials evaluating combination of oxaliplatin and 5-FU in MBC [17,18]. Considering the null hypothesis that the response rate would be 20%, at least 75 evaluable patients were required to ensure the two-sided, alpha of 0.05 and beta of 0.10 levels. Assuming 10% of dropout rate, the number of patients needed for this study was 82.
Results
1. Patient characteristics
From October 2007 to October 2009, 87 patients were enrolled from 11 centers in Korea (Fig. 1). Baseline characteristics are summarized in Table 1. The median age was 48 years (range, 30 to 71 years), and 48 patients (55.2%) had an ECOG PS status of 1. Fifty-four patients (62.1%) were HR-positive, and six patients (6.9%) were HER2-positive. Most patients (n=74, 85.1%) had visceral metastasis, and most patients (n=48, 55.2%) had more than three metastatic sites. All patients received prior chemotherapy as neo/adjuvant and/or palliative setting. All patients received prior-anthracyclines and 86 patients (98.9%) received prior-taxanes. One patient (1.1%) received three lines of chemotherapy, 34 patients (39.1%) received two lines of chemotherapy, and 45 patients (51.7%) received one line of chemotherapy in the metastatic setting.
2. Efficacy
Among 87 patients, 78 patients were available for tumor response evaluation. Four patients withdrew informed consent after one cycle of chemotherapy and five patients discontinued treatment after one cycle. Of these 78 patients, there was no CR, 30 had PR (38.5%), 23 had stable disease (29.5%), and 25 had progressive disease (32.1%). The ORR was 38.5% (95% confidence interval [CI], 26.9 to 50.0) and disease control rate was 67.9% (95% CI, 56.4 to 78.2). Median time to response in 30 patients with PR was 2.6 months (95% CI, 2.0 to 3.3).
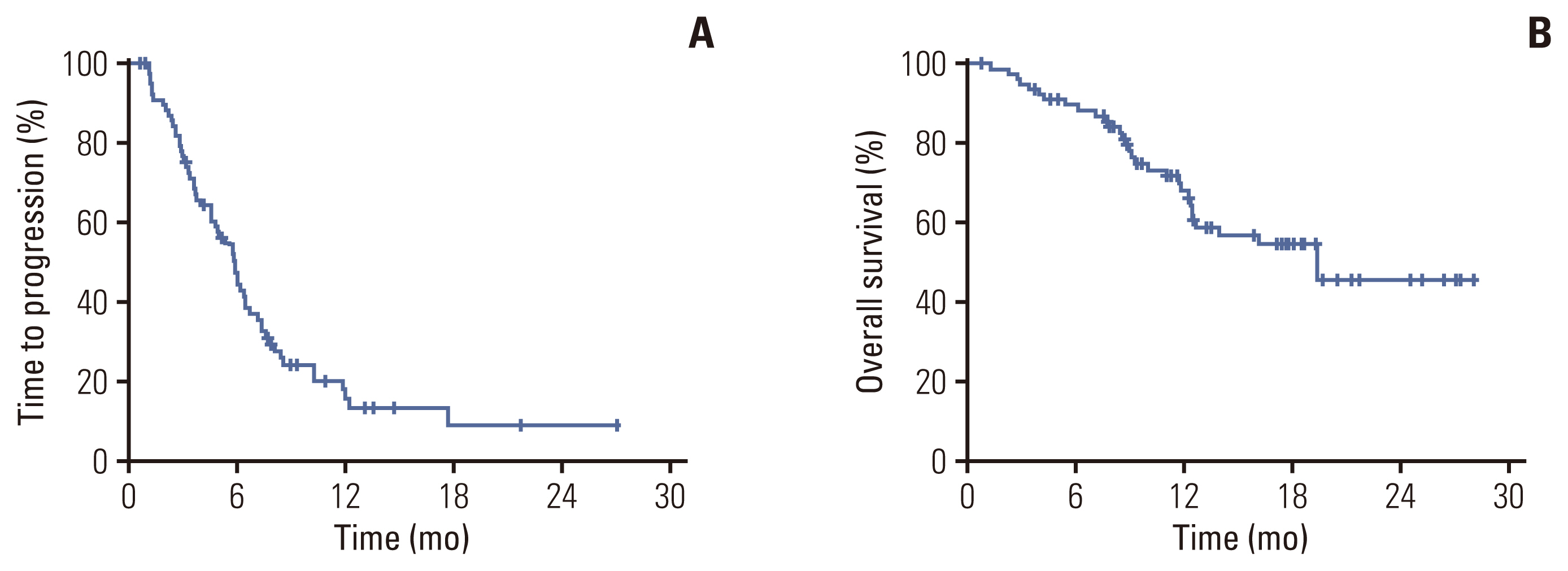
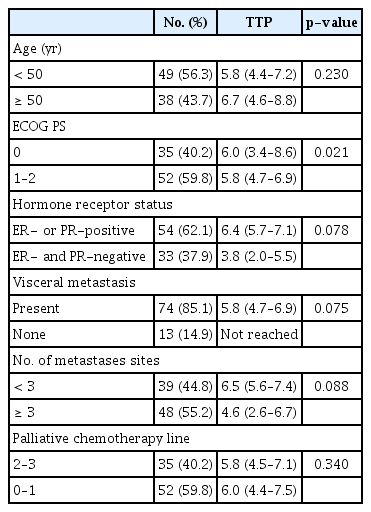
With a median follow-up of 17 months, 60 progression events and 31 death events occurred. Median TTP was 6.0 months (95% CI, 5.1 to 6.9) (Fig. 2A), median duration of response in 30 patients with PR was 10.3 months (95% CI, 5.5 to 15.1), and median OS was 19.4 months (Fig. 2B). Univariate analysis revealed ECOG PS as a prognostic factor for TTP (Table 2). Patients with HR-positive disease, those without visceral metastasis, and metastasis sites < 3 had a trend to more favorable TTP. In a multivariate analysis using the Cox-proportional hazard model in a backward stepwise method, poor performance status (hazard ratio [HR], 1.82; 95% CI, 1.05 to 3.14; p=0.032), age over 50 (HR for TTP, 1.86; 95% CI, 1.08 to 3.19; p=0.025), HR-negative disease (HR, 1.87; 95% CI, 1.07 to 3.21; p=0.027), and visceral metastasis (HR, 3.3; 95% CI, 1.16 to 9.19; p=0.025) were independently associated with a worse TTP.
3. Safety
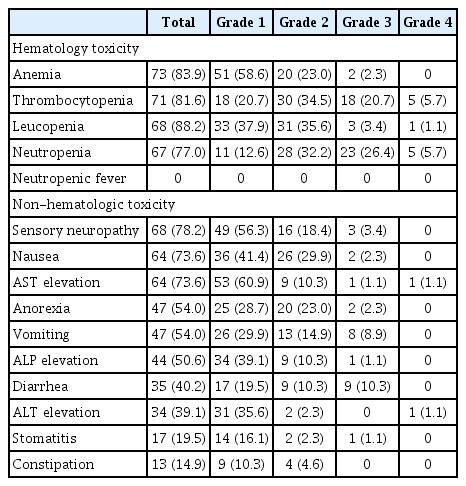
A total of 525 cycles of SOX were administered. Median number of cycles administrated to each patient was 6 (range, 1 to 22). There was at least one treatment related adverse event in 85 patients (97.7%) (Table 3). Mean dose intensity of S1 and oxaliplatin was 0.79 (standard deviation [SD], 0.18) and 0.79 (SD, 0.17), respectively. Hematologic adverse events such as anemia (82.9%), leucopenia (88.2%), neutropenia (77.0%), and thrombocytopenia (81.6%) were frequently observed. The most frequently reported grade 3 to 4 adverse event was neutropenia (n=28, 32.2%) followed by thrombocytopenia (n=23, 26.4%). However, there was no febrile neutropenia. Among non-hematologic adverse events, the most common adverse event was sensory neuropathy (78.2%), followed by nausea (73.6%), AST elevation (73.6%), anorexia (54.0%), vomiting (54.0%), and alkaline phosphatase elevation (50.6%). Only four patients (4.6%) had hand-foot syndrome (two patients with grade 1 and two patients with grade 2). Most non-hematologic adverse events were of grade 1 to 2.
Discussion
This phase II trial evaluated the efficacy and toxicity of S-1 plus oxaliplatin in patients with MBC previously treated with anthracyclines and taxanes. SOX was an effective regimen for MBC, meeting the primary endpoint with an ORR of 38.5% and a median TTP of 6.0 months. Toxicity was moderate but generally well manageable.
Although there have been much progress in endocrine therapy and HER2-directed therapy in MBC, chemotherapy still remains important armamentarium for patients with MBC. Anthracyclines and taxanes are the preferred cytotoxic agents and capecitabine, eribulin, gemcitabine, vinorelbine, platinum, and ixabepilone are commonly used in MBC refractory to anthracyclines and taxanes [2,3]. There has been a challenge to find the most feasible chemotherapeutic strategy in anthracycline- and taxane-pretreated MBC. Capecitabine is an oral fluoropyrimidine agent which is frequently used in MBC with a response rate of 20%–28% as a single agent [23–25]. S-1 is another oral fluoropyrimidine agent, which has shown similar efficacy compared to capecitabine in a phase II trial [26]. In addition, previous data from other tumors show that S-1 may have a favorable safety profile compared to capecitabine. In a meta-analysis performed in advanced gastric carcinoma, hand-foot syndrome was less prominent in S-1–based chemotherapy compared to capecitabine based chemotherapy (0.3% vs. 5.9%, p=0.003) [9]. In a randomized phase III trial performed in metastatic colorectal cancer, S-1 was associated with a significantly lower incidence of hand-foot syndrome compared with capecitabine (grade 3: 4% vs. 21%, p=0.003), with comparable efficacy [10]. In MBC, sequential single cytotoxic agents are generally preferred over combination regimen because of lesser toxicity and no significant difference in overall survival. However, combination chemotherapy regimens should be considered in case of rapid disease progression or in patients with high tumor burden. Combination regimen or fluoropyrimidines and platinum agents are frequently used in many types of tumor because of non-overlapping toxicity and synergetic anti-tumor effect [15–18]. In MBC, the combination regimen of 5-FU (intravenous) and oxaliplatin showed ORR of 27%–34% [17,18]. In a retrospective study performed in advanced triple-negative breast cancer, SOX regimen showed ORR of 34.6%, median progression-free survival of 6.7 months, and median OS of 13.3 months with a tolerable safety profile [21]. In the current phase II study, SOX regimen showed an ORR of 38.5% and a median TTP of 6.0 months. Our results suggest that SOX regimen has an efficacy comparable to the combination of 5-FU (intravenous) and oxaliplatin in MBC. Moreover, the clinical efficacy of SOX is also promising in anthracycline and taxane pre-treated MBC with high tumor burden. In the present study, all patients were pre-treated with anthracyclines and most patients were pre-treated with taxanes, and 92% of patients received palliative chemotherapy. In addition, 85% of patients had visceral metastases and more than 50% of patients had more than 3 metastatic sites.
Toxicity profile of SOX in MBC was comparable with previous reports of 5-FU (intravenous) and oxaliplatin in patients with breast cancer [17,18]. The most common non-hematologic adverse event of SOX was sensory neuropathy (78.2%) which is related to oxaliplatin. However, it was mainly of grade 1 to 2 (56.3% and 18.4%, respectively), and only three patients (3.5%) had grade 3 to 4 neuropathy. In addition, only four patients (4.6%) had hand-foot syndrome. While hematologic adverse events were frequently observed with 32.2% of patients experiencing grade 3 to 4 neutropenia, febrile neutropenia was not observed in the current trial. The toxicity profile of SOX in MBC was thus manageable. Cisplatin and carboplatin is frequently used in MBC as a single agents or in combination. In chemotherapy-naïve patients with advanced gastric cancer, SOX showed comparable efficacy with favorable safety profile compared to S-1 plus cisplatin [27]. Grade 3 or higher leukopenia (19.4% vs. 4.1%, p < 0.001), neutropenia (41.8% vs. 19.5%, p < 0.001), anemia (32.5% vs. 15.1%, p < 0.001), and febrile neutropenia (6.9% vs. 0.9%, p < 0.001) were more frequently seen in S-1 plus cisplatin than in SOX [27]. Although our study did not compare clinical efficacy between cisplatin and oxaliplatin, we could assume that the toxicity profile of oxaliplatin in MBC could be favorable compared to cisplatin. A variety of chemotherapy regimens are recommended in anthracycline and taxane pretreated MBC. Decision on serial chemotherapy regimen should be tailored to each individual patient considering performance status, disease burden, and toxicities of previous therapies. Numerous novel agents including HER2-directed therapies, CDK4/6 inhibitor, and immune checkpoint inhibitors have recently approved in the treatment of MBC. However, the accessibility are often limited in many global areas including Korea for variable reasons. We believe readily accessible SOX regimen is a reasonable option in patients with MBC as this regimen showed high response rate even in patients with high disease burden (≥ 3 metastases sites) and offers practical advantage to be administered in outpatient setting.
In conclusion, this phase II study suggests that SOX regimen is an effective regimen in heavily pretreated MBC and shows a manageable safety profile. SOX regimen may be a reasonable treatment option especially in patient with MBC refractory to anthracyclines and taxanes.
Notes
Ethical Statement
The study protocol was reviewed and approved by the institutional review board of each participating center. Written informed consent was obtained from each patient before enrollment. This study was carried out in accordance with the recommendations of the Declaration of Helsinki for biomedical research involving human subjects and the Guidelines for Good Clinical Practice (ClinicalTrial.gov Identifier: NCT00527930).
Author Contributions
Conceived and designed the analysis: Lee DW, Keam B, Im SA.
Collected the data: Lee DW, Keam B, Lee KS, Ahn JH, Sohn J, Ahn JS, Lee MH, Kim JH, Lee KE, Kim HJ, Kim SY, Park YH, Ock CY, Lee KH, Han SW, Kim SB, Im YH, Chung HC, Oh DY, Im SA.
Contributed data or analysis tools: Lee DW, Keam B, Lee KS, Ahn JH, Sohn J, Ahn JS, Lee MH, Kim JH, Lee KE, Kim HJ, Kim SY, Park YH, Ock CY, Lee KH, Han SW, Kim SB, Im YH, Chung HC, Oh DY, Im SA.
Performed the analysis: Lee DW, Keam B, Lee KS, Ahn JH, Sohn J, Ahn JS, Lee MH, Kim JH, Lee KE, Kim HJ, Kim SY, Park YH, Ock CY, Lee KH, Han SW, Kim SB, Im YH, Chung HC, Oh DY.
Wrote the paper: Lee DW, Keam B, Ock CY, Im SA.
Manuscript review and final approval: Lee DW, Keam B, Lee KS, Ahn JH, Sohn J, Ahn JS, Lee MH, Kim JH, Lee KE, Kim HJ, Kim SY, Park YH, Ock CY, Lee KH, Han SW, Kim SB, Im YH, Chung HC, Oh DY, Im SA.
Conflicts of Interest
SA Im received research fund from AstraZeneca, Daewoong Pharm, Eisai, Pfizer, and Roche outside of the current work and have been work as advisory board for AstraZeneca, Amgen, Eisai, GSK, Hanmi Corp., Lilly, MSD, Novartis, Pfizer, and Roche.
DY Oh received research fund from AstraZeneca, Novartis, Array, Eli Lilly, Servier, BeiGene, MSD, and Handok outside of the current work and have been work as advisory board for AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho, ASLAN, Halozyme, Zymeworks, BMS/Celgene, BeiGene, Basilea, Turning Point, and Yuhan.
SB Kim received research fund from Novartis, Sanofi-Aventis, and DongKook Pharm Co. outside of the current work, have been work as advisory board for Novartis, AstraZeneca, Lilly, Dae Hwa Pharmaceutical Co. Ltd, ISU Abxis, and Daiichi-Sankyo, and has stock in Genopeaks, and NeogeneTC.
JH Kim received research fund from Ono Korea Ltd outside of the current work and attended advisory board for BIXINK, Daichii Sankyo, Eisai, Lilly, Novartis, Pfizer, and Roche.
KS Lee reports grants from Dong-A Socio outside of the current work and have been work as advisory board for Eli Lilly, Novartis, Pfizer, Roche, and Bixink.
TS-1 was supported by Taiho Pharmaceutical and Jeil Pharmaceutical. Oxaliplatin was supported by Sanofi-Aventis.
Acknowledgments
We appreciated patients and their families who participated in this study.
This work was supported by a grant of the Korean National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (HA17C0055 and 1720150).