A Phase II Study of Irinotecan, 5-Fluorouracil and Leucovorin for Treatment in Patients with Previously Untreated Advanced Colorectal Cancer
Article information
Abstract
Purpose
We prospectively conducted a non-randomized phase II trial to evaluate the efficacy and safety of combination irinotecan, leucovorin (LV) and 5-fluorouracil (FU) as a first-line regimen for treating patients with previously untreated advanced colorectal cancer (CRC).
Materials and Methods
Twenty-six previously untreated patients with advanced, recurrent or metastatic CRC were enrolled in this study. The patients received either irinotecan 180 mg/m2 on day 1 with LV bolus of 200 mg/m2 and FU bolus of 400 mg/m2, and this was followed by FU continuous infusion of 600 mg/m2 on day 1 and day 2 (the FOLFIRI regimen), or they were treated with LV bolus of 400 mg/m2 and FU bolus of 400 mg/m2 followed by FU continuous infusion of 2,400 mg/m2 for 46 hours (the simplified FOLFIRI regimen), and these treatments were repeated every 2 weeks until disease progression.
Results
The objective response rate was 23.1% (6/26) respectively, for both treatments. The median time to progression was 5.3 months (range: 0.4~19.9), and the overall survival was 11.2 months (range: 0.5~52.3). The prognostic factor for longer survival was the Eastern Cooperative Oncology Group (ECOG) performance status (PS). The non-hematological toxicities were similar for both treatment groups, with more frequent grade ≥3 neutropenia being noted for the simplified FOLFIRI regimen.
Conclusion
The biweekly irinotecan based regimen was demonstrated to have a moderate antitumor activity with acceptable toxicity profiles, and the ECOG PS was the independent prognostic factor.
INTRODUCTION
In 2002, approximately 11,000 people were newly diagnosed with colorectal cancer (CRC). CRC is the fourth most frequently diagnosed cancer in Korea (1). And it has been estimated that 65,505 cancer-related death occurred in 2004, and of these 5,899 patients died due to advanced CRC (2). The prognosis of advanced CRC patients with recurrence or metastasis is dismal at best. It's been proven that palliative chemotherapy prolongs survival, increases the quality of life by easing tumor-related symptoms and improving the general well-being, and it maintains life at a high level for a longer time period compared with the best supportive care (3). Thus, chemotherapy is commonly used for advanced CRC in the palliative setting. In the past, the standard treatment of patients with advanced CRC was 5-fluorouracil (FU) that was biochemically modulated by leucovorin (LV); this has demonstrated a global response rate of 23% with a median survival that rarely exceeded 10 to 12 months (4). New trials are needed to improve the clinical outcomes. A biweekly regimen of FU, administered as a bolus and continuous infusion, combined with high-dose of LV was introduced, and this regimen obtained significantly better results for the response rate and the median progression-free survival with acceptable toxicities (5). In recent years, a number of new chemotherapeutic agents have become available. In particular, two cytotoxic agents, irinotecan and oxaliplatin, has been introduced, and the combination chemotherapy regimens that include irinotecan or oxaliplatin with FU and LV have been recommended by several studies that conducted clinical trials as the first-line treatment for advanced CRC (6~9).
Biweekly regimens such as irinotecan, LV and FU (FOLFIRI) and oxaliplatin, LV, and FU (FOLFOX) are commonly used for the treatment of CRC in clinical practice. The objective response rate of each regimen was estimated to be approximately 30 to 50% with a median survival of 15 to 20 months (6~11). We prospectively conducted a phase II trial to test the efficacy and safety of biweekly FOLFIRI regimens for the first-line treatment of previously untreated patients suffering with recurrent or metastatic advanced CRC.
MATERIALS AND METHODS
This was non-randomized, prospective, open labeled, single institutional phase II trial. In accordance with the declaration of Helsinki, the study protocol was approved by the relevant ethical committees of our institution, and a written informed consent was obtained from all the patients.
1) Eligibility
The eligibility criteria included histologically or cytologically proven, unresectable, advanced CRC. The patients had to be previously untreated for advanced disease, except for those patients in whom this therapy had been performed in the adjuvant setting at least 6 months before enrollment. Recurrent disease was defined as evident tumor progression after curative surgical resection or adjuvant treatment. The patients in this study were required to be 18 to 75 years of age, have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2 and a life expectancy of at least 3 months, and the presence of at least one uni-dimensionally measurable lesion was mandatory. Additional eligibility criteria included adequate bone marrow reserves (a white blood cell count ≥3×109/L, platelets ≥100×109/L, hemoglobin ≥100 g/L and a hematocrit ≥30%), and also adequate liver function (a total bilirubin ≤3 times the upper limit of normal; an AST and/or ALT ≤5 times the upper limit of normal) and adequate renal function (creatinine ≤1.4 ml/min).
Patients were excluded if they had any active infection, any uncontrolled central nervous system metastasis requiring emergency radiotherapy and/or corticosteroids, any serious concomitant systemic disorders, a second primary malignancy (except in situ carcinoma of the cervix or non-melanomatous skin cancers) or any severe cardiovascular disease. Any patients who were pregnant or breast-feeding were excluded from the study.
2) Treatment planning and dose modification
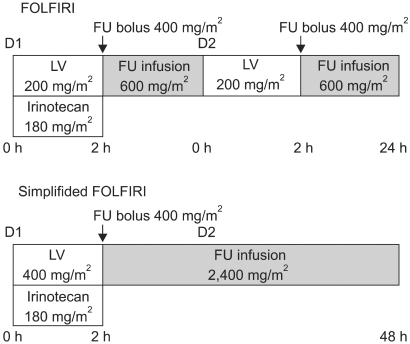
The patients were non-randomly assigned to receive either FOLFIRI or a simplified FOLFIRI regimen. The FOLFIRI regimen consisted of irinotecan 180 mg/m2 in 500 ml of 5% dextrose only on day 1, and this was concurrent with LV 200 mg/m2 that was administered as a 2-hour infusion via a Y-connector before FU 400 mg/m2 was administered as an intravenous bolus injection, and then FU 600 mg/m2 was administered as a 22-hour infusion. The LV and FU were repeated on days 1 and 2. The simplified FOLFIRI regimen consisted of the same dosage of irinotecan with the simplified administration of LV 400 mg/m2 as a 2-hour infusion before the FU 400 mg/m2 was administered as an intravenous bolus injection, and then FU 2,400 mg/m2 was administered as a 46-hour infusion (Fig. 1). Both regimens were administered at 2-week intervals until disease progression, unacceptable toxicity or stoppage by patient choice. Antiemetic prophylaxis with 5-HT3-receptor antagonist was routinely administered. Prophylactic use of granulocyte colony-stimulating factor or antibiotics was not permitted. Diarrhea or abdominal cramping or any other important symptoms of cholinergic syndrome that occurred during or within 1 hour after receiving irinotecan were treated with subcutaneous atropine 0.25 mg. For treating the symptoms of diarrhea and/or abdominal cramping that occurred more than 12 hours after receiving treatment, the patients were instructed to begin taking high-dose loperamide as soon as the first liquid stool occurred (2 mg orally every 2 hours for at least 12 hours and up to 12 hours after the last liquid stool, without exceeding a total treatment duration of 48 hours). Oral hydration with large volumes of water and electrolytes was prescribed during the whole diarrhea episode. If diarrhea persisted for more than 24 hours despite the recommended loperamide treatment, then 7-day, prophylactic, oral, broad spectrum antibiotic therapy with fluoroquinolone was initiated.
The dose adjustment schedule was evaluated at the beginning of a new course and during the previous course. It was based on laboratory analyses that were done on the scheduled day of treatment and on the day that the maximum toxicity was encountered. Chemotherapy was delayed until recovery if the neutrophils were ≥1.5×109/L, the platelets were ≥100×109/L, or for any significant persisting nonhematologic toxicity. The dose of Irinotecan was reduced to 150 mg/m2 for grades 2 to 4 neutropenia, thrombocytopenia and diarrhea. The FU bolus and infusion dose was reduced to 80% if related ≥grade 3 toxicity occurred, whereas the LV dose remained fixed.
3) Evaluation of treatment and determination of response
The clinical response evaluation procedure included all the laboratory or imaging studies that had shown abnormal findings prior to treatment. Objective tumor responses were assessed after three cycles according to the Response Evaluation Criteria In Solid Tumor (RECIST) (12). Briefly, a complete response (CR) was defined as the absence of disease at all previously known tumor sites for at least four weeks. A partial response (PR) was defined as a 30% reduction in the sum of the longitudinal diameters of all the target lesions, and this response lasted at least four weeks. Progressive disease (PD) was defined as either a 20% increase in the area of any one lesion over the prior measurement, or the development of one or more new lesions. Stable disease (SD) was defined as any change in the previous lesion that did not fit into either the PR or PD categories. All of the patients were evaluated for adverse reactions after the first dose of therapy according to the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0.
The objective response rate (RR) was defined as the best overall response for the CR or PR. Disease control was defined as the best tumor response for the CR, PR or SD that was confirmed and sustained for 4 weeks or longer. The overall survival (OS) was calculated from the date of enrollment to the date of death or to the date when the patient was last known to be alive. The time to the progression (TTP) of disease was calculated from the date of enrollment to the date of progression or death.
4) Statistics
Evaluation of the objective response was the primary end point in this trial. The objective responses were reported as relative rates. The secondary end points were TTP, OS, the disease control rate and toxicity.
The intent-to-treat (ITT) population included all the patients who were assigned and treated, and who had undergone at least one disease assessment with using the same lesion imaging procedure that was used at the study's baseline. Per protocol analysis was performed on those subjects who were eligible and assessable for response without them having incurred any major protocol deviations. The primary analysis was made on the ITT population.
The RR and disease control rate were assessed with using chi-square tests. The results were presented in terms of the estimated difference in the response rates and the p value. The OS and TTP were estimated with using the Kaplan-Meier method, and these parameters were compared with using the log-rank test (13). Multivariate analysis of the prognostic factors was performed by the Cox proportional hazard method, with the variables of age (≤60, >60 years), gender (male, female), PS (0, 1 or 2), prior adjuvant chemotherapy (relapsed, initially metastatic), the number of involved organs, liver or lung metastasis, the type of the FOLFIRI regimen that was used and the presence of salvage chemotherapy (14). Statistical significance was defined as p values ≤0.05 for the analyses. All p values were based on two-sided testing.
RESULTS
1) Patient characteristics
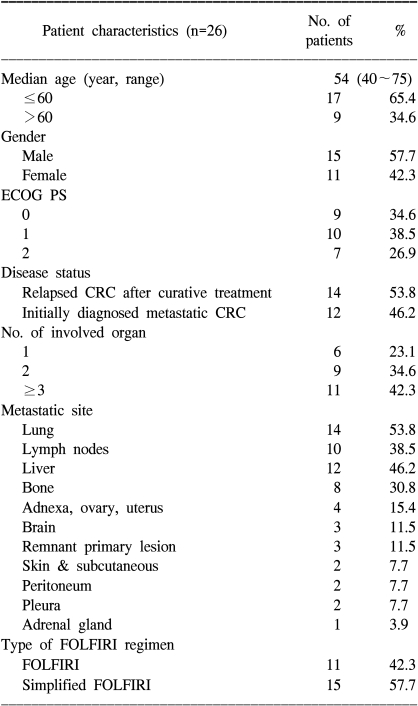
From May 2001 and April 2006, a total of 26 previously untreated patients were recruited for this trial; all of them received chemotherapy and so they were included in the safety analysis. Among the 26 treated patients, 2 patients were excluded from the response evaluation due to their discontinuation of chemotherapy after one cycle; this was attributable to consent withdrawal and lost to follow-up for one patient each. Twenty-four patients finally constituted the per-protocol population. All the reported data are for the ITT population (26 patients). The median follow-up period was 8.5 months (range: 0.5~52.3). The characteristics of the patients are shown Table 1. The median age was 54 years (range: 40~75). Fourteen patients had previously received curative therapy including surgery or adjuvant treatment. Moreover, 13 patients had received LV plus FU adjuvant chemotherapy. The relapse-free survival of those 13 patients was 15.2 months. Twenty patients had 2 or more multiple organ metastases. Eleven patients received FOLFIRI and 15 patients simplified FOLFIRI.
2) Response and survival evaluation
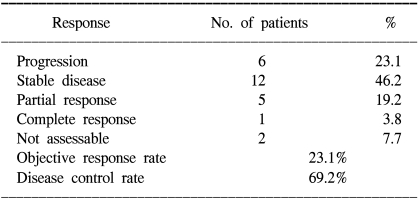
The tumor response and disease control rate in the ITT population are presented in Table 2. Five of the 26 patients (19.2%) had a PR with one CR (3.8%). The RR was 23.1% (95% CI: 6.1~40.1). Although the RR for the FOLFIRI treated patients was higher than that for the simplified FOLFIRI treated patients (9.1 vs 33.3%, respectively), the RR of the two groups did not statistically differ (p=0.098). The observed disease control rate was 69.2%, and each disease control rate was 71.7% and 66.7% for the patients who received FOLFIRI and the simplified FOLFIRI, respectively (p=0.813).
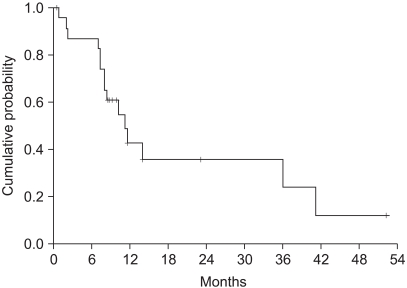
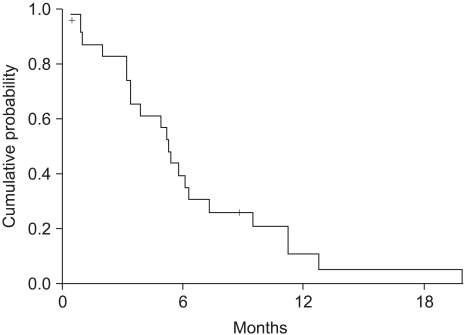
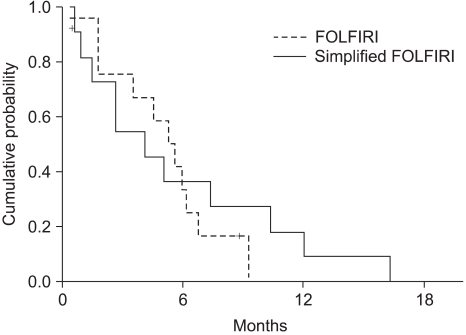
The median survival was 11.2 months (range: 0.5~52.3, 95% CI: 7.4~15) (Fig. 2). The independent prognostic factor for improved survival was a good PS (p=0.001) (Fig. 3). The survival of the ECOG PS 0 patients did not reach to the median value, and the median survival of patients with an ECOG PS of 1 and 2 were 10.1 months and 7.3 months, respectively. Survival of the patients was not affected by other factors such as gender, age, prior adjuvant chemotherapy, the number of involved organs, liver or lung metastasis, the type of FOLFIRI and the salvage chemotherapy. The median TTP was 5.3 months (range: 0.4~19.9, 95% CI: 4.5~6.1) (Fig. 4). Any independent factors with statistically significance for improving the TTP were not detected.
3) Toxicity
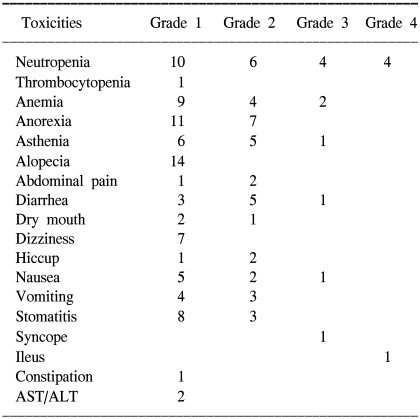
All patients were assessable for toxicity. A total of 194 cycles were performed, and there were no therapy-related deaths. The median number of cycles was 7 (range: 1~18). Table 3 presents the patients who experienced treatment-related toxicities of NCI-CTCAE. The frequently observed toxicities were grades 1 and 2. A higher incidence of neutropenia was recorded for the patients who received the simplified FOLFIRI than for those patients who received FOLFIRI. Grade 3~4 neutropenias were more frequent for the simplified FOLFIRI treated patients. The incidence of the other grades 1~4 non-hematological and hematological toxicities were similar in both treatment groups.
DISCUSSION
This study reports on conducting a trial for determining the efficacy of biweekly FOLFIRI regimens as the first-line treatment for patients suffering with advanced CRC. Although this trial was initially started with a statistically calculated target sample size, many reasons such as the slow speed of enrollment and the paradigm shift of treatment interfered with our plan.
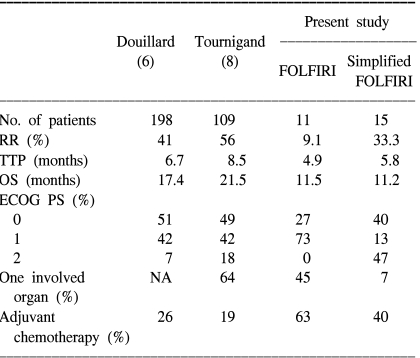
For the last few decades, FU-based chemotherapy with LV has remained the mainstay of treatment of CRC patients. In recent years, two new cytotoxic agents, irinotecan and oxaliplatin, have been introduced, and combination chemotherapy regimens that include irinotecan or oxaliplatin with FU and LV have been recommended by clinical trials as the first-line treatment for advanced CRC (6~9). The FOLFIRI and FOLFOX regimens demonstrated superiority for survival, TTP and the quality of life, compared to the FU and LV combination regimen. Our results are relatively unsatisfactory in that the RR was 23.1%, and the median survival and TTP were 11.2 and 5.3 months, respectively. These reasons for this were thought to be that the proportions of patients with recurrent disease after curative treatment, including adjuvant chemotherapy, were higher than that of the other previous trials. About 53% of the patients had been treated by adjuvant chemotherapy and then they relapsed. Also, our trial included a greater number of patients with a poor PS and multiple organ metastases. Previous adjuvant chemotherapy and a poor PS, and also multiple organ disease are all thought to be unfavorable prognostic factors for the treatment of advanced CRC. Our study also demonstrated that PS was an independent prognostic factor for survival. These considerations might explain the relatively lower RR, OS and TTP that were observed in our study compared with the results of other previous studies. In Table 4, our results are compared with those of the previous studies.
There are preclinical in vitro data, as well as clinical in vivo data suggesting that FU has a schedule-dependent mechanism of action (15). When this agent is delivered by a short-duration bolus injection, then it mainly inhibits RNA synthesis. When FU is delivered via a long-term infusion lasting days to weeks, then it mainly inhibits DNA synthesis through the inhibition of thymidylate synthesis. Historically, treatment with bolus FU has led to modest response rates of approximately 12%, and also a median survival of approximately 11 months. Continuous infusion of FU has shown better results in terms of the response rate and OS than has bolus FU (16). A hybrid regimen of FU (LVFU2) with a bolus and then an infusion administration of FU obtained significant improvements in the response rate and TTP compared with the standard low-dose LV plus FU bolus schedule of the North Central Cancer Treatment Group regimen (5). In a phase II study that combined LVFU2 with irinotecan for treating CRC patients, this regimen was well-tolerated and there was an increased response rate, a longer time to progression and better survival, with a later deterioration of the quality of life (6). A simplified LVFU2 regimen that combined LV plus FU bolus on day 1 only with a high dose FU infusion was introduced next. This simplified biweekly regimen showed low toxicity and it was less expensive than LVFU2 and more convenient to the patient than the other LV plus FU regimen (17). In another phase III study for patient with untreated CRC, biweekly irinotecan with simplified LVFU2 demonstrated promising results, which were 56% for the RR, 21.5 months for the OS, and 8.5 months for the TTP (8).
To verify and compare the activity of these two regimens, we started our protocol in 2001. Twenty-six patients were entered onto this trial and they were non-randomly assigned to receive either the FOLFIRI regimen according to Douillard et al (6) or the simplified FOLFIRI regimen according to Tournigand et al (8).
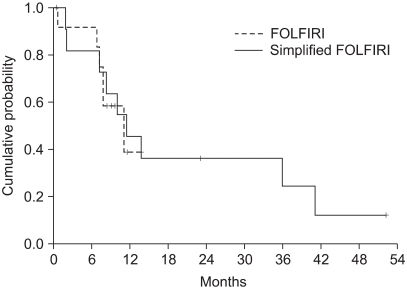
Although we would like to compare the efficacy of the two biweekly FOLFIRI, any differences were not detected. The results showed that no difference, in term of the RR, was observed for the two regimens, with the objective responses being 9.1% for the FOLFIRI arm and 33.3% for the simplified FOLFIRI arm (p=0.098). Also, the OS and TTP did not statistically differ between the two regimens (Fig. 5, 6). We think that these figures were the result of the weak points of our study, which are that this was a non-randomized trial, there was a small sample size for each arm, and there was an imbalance between the numbers of patients in the two treatment groups.
CONCLUSIONS
This study has demonstrated that the efficacy of biweekly FOLFIRI and combination therapies seemed effective as first-line treatments for advanced CRC patients. Our results confirmed the moderate efficacy of adding irinotecan to the LV and FU, and also the mild toxicity of these regimens. Well designed, large scale phase 2 or 3 trials and further studies about the addition of targeted agents to these cytotoxic regimens are needed in the future.