Current Status and Challenges of Cancer Clinical Trials in Korea
Article information
Abstract
Purpose
Cancer clinical trials in Korea have rapidly progressed in terms of quantity and quality during the last decade. This study evaluates the current status of cancer clinical trials in Korea and their associated problems.
Materials and Methods
We analyzed the clinical trials approved by the Korea Food and Drug Administration (KFDA) between 2007 and 2013. A nationwide on-line survey containing 22 questions was also performed with several cooperative study groups and individual researchers in 56 academic hospitals.
Results
The number of cancer clinical trials approved by the KFDA increased almost twofold from 2007 to 2013. The number of sponsor-initiated clinical trials (SITs) increased by 50% and investigator-initiated clinical trials (IITs) increased by almost 640%. Three hundred and forty-four clinical trials were approved by the KFDA between 2012 and 2013. At the time of the on-line survey (August 2013), 646 SITs and 519 IITs were ongoing in all hospitals. Six high volume hospitals were each conducting more than 50 clinical trials, including both SITs and IITs. Fifty-six investigators (31%) complained of the difficulties in raising funds to conduct clinical trials.
Conclusion
The number of cancer clinical trials in Korea rapidly increased from 2007 to 2013, as has the number of multicenter clinical trials and IITs run by cooperative study groups. Limited funding for IIT is a serious problem, and more financial support is needed both from government agencies and public donations from non-profit organizations.
Introduction
In 2010, 202,053 cases of cancer were diagnosed, there were 72,046 deaths due to cancer, and one out of four patients died of cancer in Korea [1]. Even with the development of early screening and diagnosis technology, more than half of cancer patients are diagnosed in advanced stages when curative surgical resection may not be feasible, and additional chemotherapy is often required. Palliative chemotherapy has significantly improved the overall survival of cancer patients [2]. Furthermore, cancer therapeutics have advanced beyond simply killing or inhibiting cancer cell growth by cytotoxic anticancer drugs, to targeting intracellular or intercellular cancer-specific signaling pathways, so called “targeted agents,” according to molecular genotype. The anticancer drug market reached 62 billion dollars worldwide in 2012 and is rapidly increasing every year [3]. In this era of evidence-based medicine, clinical trials are essential for the establishment of standardized treatments or treatment guidelines for cancer therapy. In the United States, clinical trial cooperative groups for multicenter trials such as the Cancer and Leukemia Group (CALGB) were launched in 1956 and are mainly supported by the National Cancer Institute or industry [4]. Large investigator-initiated clinical trials (IITs) led by these cooperative groups led to rapid improvement in cancer treatment over the last several decades. In Korea, the anticancer market reached 3 trillion Korean won in 2010, but most drugs were products of multi-national pharmaceutical companies and only a few domestic pharmaceutical companies in Korea are involved in the early developmental stages of new anticancer drugs [5]. During the last few decades, Korean investigators have been participating in multi-national clinical trials led by multi-national pharmaceutical companies, due to relatively low expenses and excellent human resources. The number of Korea Food and Drug Administration (KFDA) registered investigational new drug clinical studies numbered only 45 cases in 2001, but increased to over 670 cases in 2012 [6]. In addition, the Korea National Enterprise for Clinical Trials was established in 2007 and was assigned to 15 regional clinical trial centers. The main goal was to expand the clinical trial infrastructure for new drug development through collaboration with government, academic societies, and industry. However, national support for IIT remains significantly lower than in other countries including the United States, Europe, and Japan. To improve public health through collaborative cancer studies, several cooperative clinical trial groups including the Korea Cancer Study Group (KCSG), Korean Radiation Oncology Group (KROG), and Korean Gynecology Oncology Group (KGOG) were established and are actively involved in IIT. This study evaluates the status and problems of cancer clinical trials in Korea.
Materials and Methods
The status of sponsor-initiated cancer and investigatorinitiated cancer clinical studies was evaluated using a questionnaire in cooperation with oncology study groups such as the KCSG, KGOG, and KROG. This questionnaire consisted of sponsor source, researcher behavior, single institute vs. multi-institute study, study purpose, phase of clinical trial, intervention model and method, masking, allocation, enrollment target number, direction and timing, type of intervention, difficulties of cancer clinical trials, and clinical trial resources. Investigators were surveyed nationwide in detail from 56 academic hospitals in Korea on matters regarding cancer clinical trials in Korea. Questions were asked through an on-line survey using a cross-sectional study design in August 2013.
We analyzed the characteristics of the clinical trials approved by the KFDA between 2007 and 2013 and evaluated the clinical trials approved by the KFDA between 2012 and 2013 using the on-line questionnaire items listed above [7].
Results
1. Cancer clinical trials approved by the KFDA
The number of total and cancer clinical trials approved by the KFDA increased from 2007 to 2013. During that period, the number of cancer clinical trials approved by the KFDA increased by almost 100%. The number of sponsor-initiated clinical trials (SITs) increased by 50% and IITs increased by almost 640% between 2007 and 2013. Fig. 1 shows the annual number of total and cancer clinical trials approved by the KFDA. In 2012, 679 clinical trials were approved by the KFDA and 27.5% of trials (187/679) were cancer clinical trials. One hundred and twenty-one trials were SITs and 66 were IITs. In 2013, 157 cancer clinical trials were approved out of a total 607 clinical trials, including 105 SITs numbered and 52 IITs. The characteristics of clinical trials approved by the KFDA between 2012 and 2013 are in Table 1. The types of cancers investigated by SITs, in order of increasing number, were lung, breast, lymphoma, hematology, hepatobiliary-pancreas, and stomach (Fig. 2A). In contrast, the types of cancers investigated by IITs, in order of increasing number, were lung, lymphoma, breast, stomach, hepatobiliary-pancreas gynecological, and genitourinary tract (Fig. 2B).
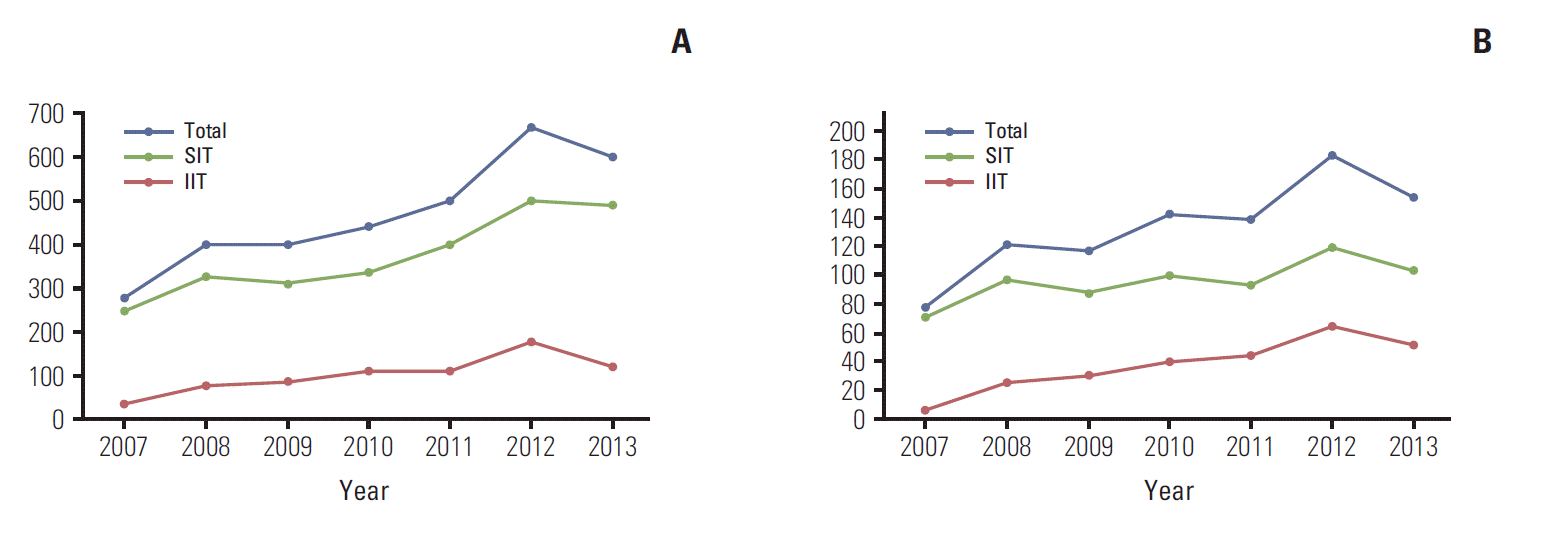
Clinicaltrials approved by Korea Food and Drug Administration (KFDA) annually.(A) Total clinicaltrials.(B) Cancer clinical trials. SIT, sponsor-initiated clinical trial; IIT, investigator-initiated clinical trial.
2. Cancer clinical trial questionnaire results
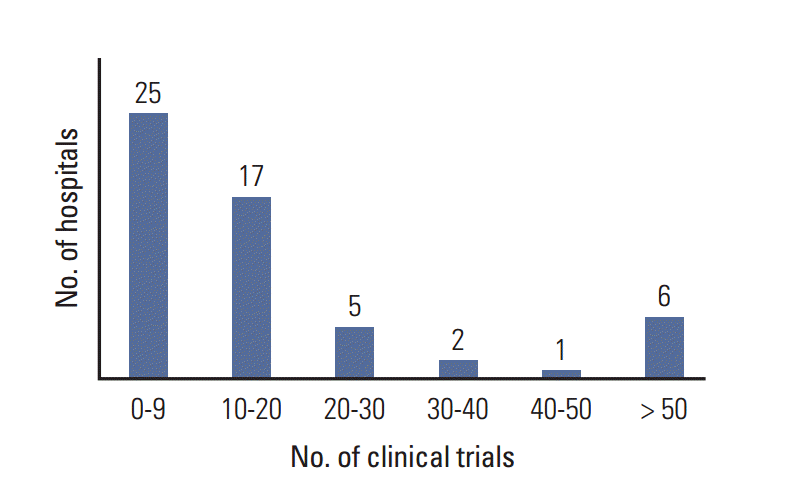
Questionnaires were obtained from the oncology departments of 56 hospitals, the obstetrics and gynecology departments of five hospitals, the surgery departments of three hospitals, and the radiation oncology department of one hospital. Multicenter clinical trials were counted multiple times because we could not avoid an overlapping count of multicenter trials from individual hospitals. In the 56 hospitals, 646 SITs and 519 IITs were ongoing, including both interventional and observational studies. The number of hospitals according to the number of clinical trials is shown in Fig. 3.
Among 519 IITs, there were 393 interventional studies and 126 observational studies. The characteristics of the currently ongoing clinical trials are in Table 2. One hundred and fifty-nine trials (18.4%) among 866 were multidisciplinary clinical trials.
3. Difficulties in conducting cancer clinical trials
Sixty-six investigators (36.7%) complained of difficulties with human resource management and 56 (31%) complained of difficulties with fund-raising for clinical trials. Thirty-two investigators (17.8%) experienced difficulties in collaborating with other hospital departments and 15 (8.3%) complained of the difficulties in communication with the Institutional Review Board (IRB). Other problems included the complexity and tediousness of administrative procedures.
4. Cancer clinical trial resources
Among 56 surveyed hospitals, 45 hospitals had a clinical research center. Only 18 hospitals employed clinical research coordinators (CRCs) under the name of the institution, so in the remaining institutions, individual investigators hired their own CRCs. Thirty-six hospitals had 1-3 CRCs, six hospitals had 4-6, and six hospitals had more than 6 CRCs. In contrast, eight hospitals did not have CRCs. Twenty-two 22 hospitals held regular meetings of IRBs once a month, 21 hospitals held meetings twice a month, and 13 hospitals held meetings more than twice a month. Thirteen hospitals did not have IRB internal audits, 24 hospitals had one, and 19 hospitals had two or more. Among 56 hospitals, five hospitals had an independent data manager in their clinical trial center, and 31 hospitals had a separate facility for sample storage for clinical trials.
5. Cooperative study groups for multicenter cancer clinical trials in Korea
Domestic multicenter clinical trial organizations such as the KCSG, KROG, and KGOG actively conduct clinical trials. In 2013, there were 27 multicenter clinical trials conducted by the KCSG, 11 by the KROG, and 17 by the KGOG.
Discussion
Cancer clinical trials are vital to determining treatment guidelines for cancer patients, to evaluate the safety and efficacy of new treatments, and to establish new evidence.
To conduct clinical trials in the United States, 10 clinical trial cooperation groups comprised of more than 3,100 institutions were launched and supported by the National Cancer Institute [8]. These 10 clinical trial groups include the Cancer and Leukemia Group, Children’s Oncology Group, Eastern Cooperative Oncology Group, North Central Cancer Treatment Group, Southwest Oncology Group, American College of Surgeons Oncology Group, American College of Radiology Imaging Network, Gynecologic Oncology Group, National Surgical Adjuvant Breast and Bowel Project, and Radiation Therapy Oncology Group. Both medium-sized phase 2 clinical trials and large-scale phase 3 clinical trials are being conducted and 25,000 patients per year participate in clinical trials [8]. These clinical trials could result in important outcomes that could change clinical practice directly related to patient treatment guidelines. Conversely, single-center studies or IITs are small-scale designs such as nonrandomized phase II studies that usually do not result in changes in clinical practice.
The number of cancer clinical trials approved by the KFDA increased almost twofold from 2007 to 2013. In particular, IITs rapidly increased by almost 640% between 2007 and 2013. The rapid growth of IITs is mostly attributed to increased interest in clinical trials by motivated Korean investigators and by the efforts of multicenter clinical trial organizations such as the KCSG, KROG, and KGOG. Both SITs and IIT are actively involved in studies of lung, breast, lymphoma, stomach, and hepatobiliary-pancreatic cancer.
Fig. 3 shows that several large volume hospitals are conducting more than 50 clinical trials each. Most investigators complained of difficulties with human resource management and obtaining financial support for clinical trials due to unequal distribution of clinical trials. Thus, multicenter clinical trial groups such as the KCSG and governments should support individual investigators, especially small volume hospitals actively participating in clinical trials, to establish the infrastructure for clinical trials.
The KFDA approved 226 SITs and 118 IITs between 2012 and 2013. Fifty-six hospitals were conducting 646 SITs and 519 IITs at the time of the on-line survey. SITs are usually supported by pharmaceutical companies, and contact research organizations also conduct a large proportion of cancer clinical trials. In contrast, some IITs have difficulty with standard operating procedures for clinical trials because of limited human resources and are not supported by pharmaceutical companies or government, quite different from the situation in western countries where there is government support. For these reasons, relatively small-sized single-center clinical trials are common, leading to low academic value and the high possibility of error. In addition, most clinical trials are phase 2 and 3 clinical trials, but the number of phase 1 clinical trials is very low due to their high cost and complexity.
From a public health standpoint, industry-sponsored cancer clinical trials may be unduly influenced by the pharmaceutical industry. Although many SITs lead to changes in standard clinical practices, pharmaceutical companies have less interest in improving standard treatments related to conventional cancer diagnosis, treatment, and prevention, or reducing economic loss due to unnecessary treatments. Conversely, investigator-initiated multicenter cancer clinical trials are more concerned with a scientific approach such as clinical trials, which can improve or change clinical practice or reduce national medical costs. One limitation of our study is the questionnaire. Questionnaires have inherent inaccuracy and in our case, an unavoidable overlapping count of multicenter trials from individual hospitals. Therefore we also examined status of cancer clinical trials though the evaluation of the clinical trials approved form the KFDA for overcoming these limitations.
In many countries, the standard treatment costs for IITs are usually covered by health insurance. However, a consensus on supporting costs for standard of care in IITs has not been reached in Korea. Additionally, most IITs in Korea require approval from the KFDA, but investigators and institutions are responsible for conducting clinical trials from start to finish. This process of approval entails expense and time that is borne by the investigators.
In Korea, multicenter clinical trial groups such as the KCSG, KROG, and KGOG conduct a variety of clinical trials. Several lines of evidence have been established through multicenter clinical trial groups. For example, phase 3 clinical trials of gefitinib versus pemetrexed as second-line treatments in non-small cell lung cancer and maintenance chemotherapy versus observation after achieving disease control with six cycles of gemcitabine plus paclitaxel in breast cancer were supported by the KCSG [9,10].
Therefore, it is clear that these types of multicenter clinical trial organizations are desirable and encourage the development and activation of IITs. Moreover, support is urgently needed from the relevant governments, study groups, and organizations for the promotion of multicenter IITs. Furthermore, to improve the quality of multicenter clinical trials, support is needed for the establishment of infrastructure such as data management, statistics, and data monitoring. Long-term supported projects such as the “Cancer Overcome Project” run by the National Cancer Center in Korea, will ultimately contribute to public health improvement by providing new evidence for the improvement of treatment outcomes and the establishment of cancer treatment guidelines.
Conclusion
The number of cancer clinical trials in Korea rapidly increased from 2007 to 2013, as has the number of multicenter clinical trials and IITs run by cooperative study groups. For more development and more activation of IITs, active support is urgently needed from the relevant governments, study groups, and organizations.
Notes
Conflict of interest relevant to this article was not reported.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (Issue No.1320390).
The authors are indebted to questionnaire respondents, as well as to all members of the KCSG, KROG, and KGOG.