Irradiation of Donor Mononuclear Cells for Treatment of Chemorefractory Metastatic Solid Cancers: A Community-Based Immune Transplant Pilot Study
Article information
Abstract
Purpose
Chemotherapy has demonstrated ability to generate tumor antigens secondary to induction of apoptosis, against which human leukocyte antigen-compatible, irradiated, related donor mononuclear cells may be administered with immune stimulation to activate antigen presenting and cytotoxic T cells, while minimizing risk of graft-versus-host disease (GVHD). The present study endeavours to describe feasibility and efficacy of this treatment, specifically in the community setting.
Materials and Methods
Eligible patients had rapidly progressive, chemorefractory metastatic solid tumors. Treatment consisted of intravenous etoposide and cyclosporine for three days followed by granulocyte-macrophage colony-stimulating factor for 5 days. The following week, 5×107 haploidentical or more closely matched irradiated donor mononuclear cells were given weekly for 10 weeks along with interleukin-2.
Results
Three patients were enrolled, and the regimen was well-tolerated, with no GVHD observed. All patients had clinical response, despite advanced and heavily pretreated disease.
Conclusion
The above-outlined protocol demonstrates favorable tolerability and efficacy, and appears to be feasible in the community setting. While the optimal chemotherapy, immunostimulation, and irradiation regimens may be further optimized, future investigation appears warranted, and may include community oncology programs.
Introduction
While chemotherapy-refractory metastatic solid tumors generally portend poor outcomes, anecdotal evidence suggests that some longer-than-anticipated survivors develop an immune response against the cancer [1-4]. For example, lung cancer patients with increased concentration of cytotoxic (CD8) T cells specific for tumor demonstrate superior survival compared with lower levels (of cytotoxic T cells) [1,2]. Similarly, increasing duration of survival was noted in patients with metastatic melanoma who demonstrated increased concentration of CD8 T cells directed against their tumor [3,4].
Most cancer patients have some CD8 T cells directed against their tumor antigens, however, overall they are tolerant of their cancer and display a limited immune rejection of their tumor [5]. "Helper" (CD4) T cells, needed to help stimulate the growth of CD8 T cells as well as having direct action against tumor cells, have been more difficult to find and have not been functional in cancer patients [6]. Also, antigen presenting cells (APCs), necessary for activating helper and cytotoxic T cells, in cancer patients are suppressed as well [7]. All of these cell types are necessary for an individual to successfully attack counteract a cancer; however, these are not effective in patients with advanced cancer [8]. In addition, regulatory cells, which usually prevent autoimmune disease, can blunt the immune response to tumor cells, which can look, to the patient's immune system, like normal cells [9]. These regulatory cells appear to be increased in cancer patients [10].
Surprisingly, evidence from studies in human cancer strongly suggests that the tolerance against cancer cells can be broken by the temporary administration of donor mononuclear cells [11,12]. In one non-myeloablative transplant regimen for patients with refractory myeloma or lymphoma, patients received thymic irradiation, antithymocyte globulin, and cyclophosphamide followed by two mononuclear cell infusions (5 weeks apart) [11]. One third of patients experienced total loss of donor cells yet 41% of these same patients achieved and objective response and 20% of these were complete responders, despite the loss of donated cells. A similar phenomenon has been observed with other different nonmyeloablative transplants [12].
Unfortunately, breaking tolerance to cancer by adding donor mononuclear cells carries the risk of graft-versus-host disease (GVHD) [13]. This can be prevented by irradiating the mononuclear cells, and investigations employing multiple doses of irradiated mononuclear cells to break tolerance to the cancer have been successful without any GVHD [13]. This pilot study sought to explore preliminary data of adding chemotherapy and cytokine therapy to irradiated mononuclear cell infusion to patients with advanced cancer without further standard options for treatment.
Materials and Methods
1. Patients and donors
Following Institutional Review Board (IRB) approval, eligible patients were enrolled from August 2001 to December 2004 at MedCenter One Department of Hematology and Oncology (Bismarck, North Dakota). Written informed consent was obtained from all eligible patients and their donors before participation in this trial.
Initial tests were performed on patients within 28 days before the start of the treatment, and included complete medical history, complete review of systems, and complete physical examination, computed tomography scans of the chest, abdomen, and pelvis, blood chemistry including liver profile, complete blood count (CBC) with differential, and electrocardiogram, blood type and serologic human leukocyte antigen (HLA) typing and tests for previous cytomegalovirus (CMV) infection. Initial tests performed on the donors included complete history and physical performed by an outside personal physician, blood typed and crossmatched with the recipient as well as tests for standard blood donors including previous hepatitis B or C infection, and previous infection with human immunodeficiency virus or human T-lymphotropic virus type I, CMV, syphilis, West Nile virus, or Trypanosoma cruzi.
Eligibility criteria included patients with measureable or clinically evaluable metastatic solid tumor who had received standard treatments and had progressive disease over a 3 month period. Pathologic confirmation of the primary tumor was required. Patients were required to be older than 18 years, with normal marrow function (absolute neutrophil count of >1,000/mm3, hemoglobin>8.0, and platelets >100,000/mm3), adequate liver function (aspartate aminotransferase<2× normal institutional values and total bilirubin<2 mg/dL), and adequate renal function (creatinine<2.0). In addition, patients were required to have adequate cardiac function (ejection fraction of ≥40%) and a Karnofsky performance status of >50%. Patients with brain metastases or who had other severe uncontrolled medical problems were excluded.
Donor eligibility included relatives aged ≥18 years who shared at least one set of HLA class I antigens with the patient (i.e., at least haploidentical with the patient and meet standard donation requirements for blood donation according to the American Association of Blood Banks; http://www.aabb.org/resources/bct/Pages/aabb_coi.aspx). Donors had to be in good general health, available and able to donate on an apheresis machine for 3 to 4 hours weekly for 10 weeks for infusion at Medcenter One. Absolute donor exclusion criteria were donors who are homologous for an HLA class I antigen for which the patient (donor recipient) was heterozygous, or donors who have been infected with the CMV if the patient demonstrated no evidence of past infection.
2. Interdepartmental coordination
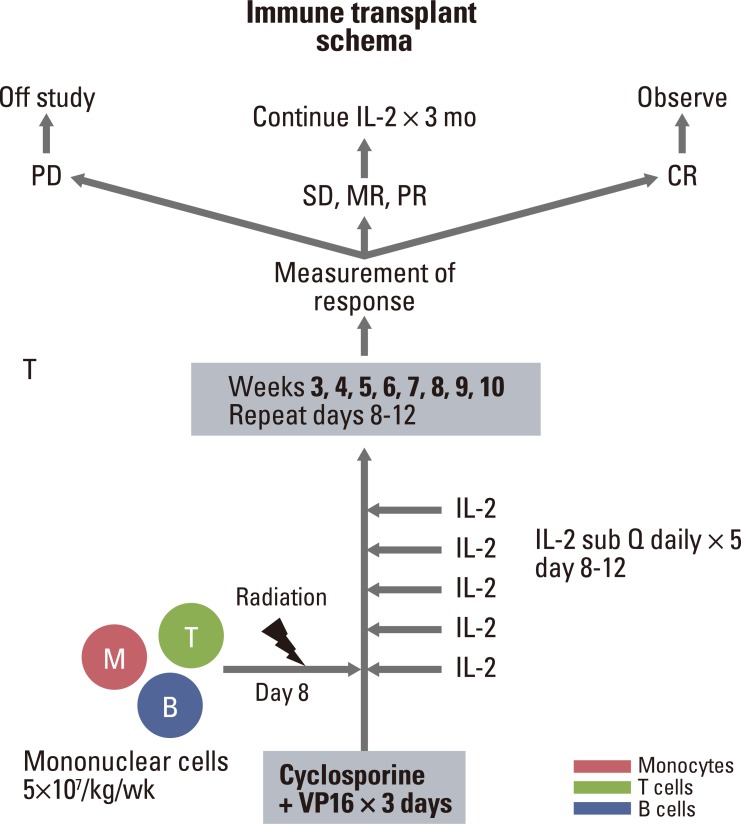
This protocol required coordination between four different departments: the renal department performed aphaeresis, while the blood bank processed the mononuclear cells and ensured that the donor cells were acceptable for infusion. This often required hand processing to remove cell clumps, as well as counting and removing excess cells from the bag to meet the required donor cell amount of 5×107/kg, all in a closed system. Thereafter, the transfusions were typed and crossed against the patient's blood. Overall, processing was accomplished in approximately 30 minutes; thereafter, the cells were transported to the radiation therapy department. Irradiation of the cells was accomplished in approximately 5 minutes, performed in between regularly scheduled patients, with a special holder for the collection bag designed by our physics department for external beam radiation of blood products. Including transportation, the time required was approximately 30 minutes to irradiate the mononuclear cells and deliver them to the oncology floor for infusion. Infusion of the mononuclear cells was performed over approximately 15 minutes. Time from the end of collection to infusion averaged approximately 60 minutes, and from start of apheresis to infusion averaged 4 to 5 hours. Pre-medication consisted of acetaminophen only. Patients were able to be treated as an outpatient and could leave the hospital before 5 PM on the same day every week to minimize staff scheduling conflicts. A protocol scheme is demonstrated in Fig. 1.
3. Treatment regimen
All patients received cyclosporine 1 mg/kg over 4 hours followed by VP-16 100 mg/m2 over 60 minutes on the first day. If the patient tolerated the cyclosporine well, on the second and third day, cyclosporine 1 mg/kg was given over 2 hours followed by VP-16 100 mg/m2 over 60 minutes. This was to produce tumor cell apoptosis and release of tumor antigen, similar to the murine model. After chemotherapy, granulocyte-macrophage colony-stimulating factor (GM-CSF) at 500 µg was given subcutaneously once daily for 6 days, in order to enhance tumor antigen processing and presenting.
In the second week, mononuclear cells 5×107 kg/wk were collected from the donor and delivered to the patient after being processed in a closed system in the blood bank and irradiated with 2,500 cGy (initially) in the radiation therapy department. They were delivered to the patient as soon as possible after collection and monitored according to standard marrow infusion criteria. These were to act as antigen processing and presenting cells, helper cells, and cytotoxic T cells and natural killer (NK) T cells to stimulate the patient's immune response against the cancer. Along with the irradiated mononuclear cells, interleukin (IL)-2 was administered daily from Monday to Friday subcutaneously at a dose of 4.5×106 IU/m2/day to stimulate the development of cytotoxic T cells and helper T cells. Of note, following enrollment of the first two patients, a protocol modification was requested of and granted by the IRB to permit radiotherapy dose reduction to 750 cGy. This was done in an effort to permit greater mononuclear cell efficacy [13], as our system did not permit escalation of the weekly dose of mononuclear cells.
Irradiated mononuclear cells were continued weekly from week 2 to week 10, IL-2 was also continued weekly along with the mononuclear cell infusions. Dose adjustments of IL-2 were allowed for acute and chronic toxicity. Following enrollment of the first two patients, a formal protocol modification was requested of and approved by the IRB, to decrease the dose of IL-2 to 7.5×106 IU given three times per week, in an effort to improve tolerability. Up to one alternate donor was allowed who meet all the required criteria in the event that a particular donor was not available.
Patients were evaluated weekly before receiving donor mononuclear cells for signs of toxicity from the treatment during the treatment phase of the study. After 12 weeks of treatment, patients were evaluated every 2 weeks for toxicity. The toxicity criteria were according to the National Cancer Institute Common Toxicity Criteria ver. 2. Patients weekly received focused physical examinations including vital signs, weight and complete review of systems, complete blood chemistries including serum bilirubin, alanine and asparate aminotransferase, protein, albumin, creatinine, blood urea nitrogen, sodium, potassium, chloride, calcium, glucose, carbon dioxide, albumin and total protein, and CBC with differential. Donors were evaluated weekly including vitals, physical examinations and review of systems, complete blood chemistries, and CBC with differential, before being allowed to donate.
4. Response assessment
Disease response was measured after 12 weeks of treatment and every 2 months thereafter. Continued IL-2 was permitted if a response was seen after 12 weeks of treatment. Response by imaging was not an endpoint in this study, however the Response Evaluation Criteria in Solid Tumors (RECIST) was used to evaluate responses and determine progression in patients with measureable disease. A separate category of mixed response was included in this study to accommodate a signal of some promise (i.e., mixed response was defined as meeting criteria for progression, yet showing some indication of disease response such as slower disease progression, significant decrease in symptoms, one lesion which resolved, or some lesions becoming smaller while others becoming larger).
5. Toxicity scoring and dose escalation
Dose-limiting toxicity (DLT) was defined as grade 3 toxicity due to the mononuclear cells, not due to IL-2. Also, GVHD of any grade was defined as DLT in this pilot study. If no patient experienced grade 2 toxicity after the first two patients, then the number of irradiated mononuclear cells would be increased to 1×108 (infused weekly). If DLT was experienced, the first stage of the trial would cease, and trial would move to stage II, as per study design. The maximum tolerated dose was defined as the dose at which one (or no) patient experienced DLT, with the next higher dose demonstrating DLT in two out of six patients.
6. Study objectives
The primary study objective was to determine whether mononuclear cells could be collected weekly from a haploidentical or more closely matched donor, processed in the blood bank, irradiated, and administered in the same day in a community hospital environment (requiring coordination between the apheresis department, the blood bank, radiation therapy, and oncology). Secondary objectives included establishing the initial toxicity data of irradiated mononuclear cell infusions given along with low dose IL-2 after standard dose chemotherapy, determination of a practical dose of irradiated mononuclear cells which could be given weekly, and evaluation of the efficacy of this regimen in terms of patient response and survival.
Results
1. Enrollment
A total of 3 patients were enrolled in this pilot study. Each patient had advanced disease, refractory to multiple courses of chemotherapy, and was eligible for hospice but desiring further intervention. A summary of each patient's course is described below. The first 2 patients received a target dose of 5×107 mononuclear cells/kg irradiated with 2,500 cGy to prevent GVHD.
2. Patient 1
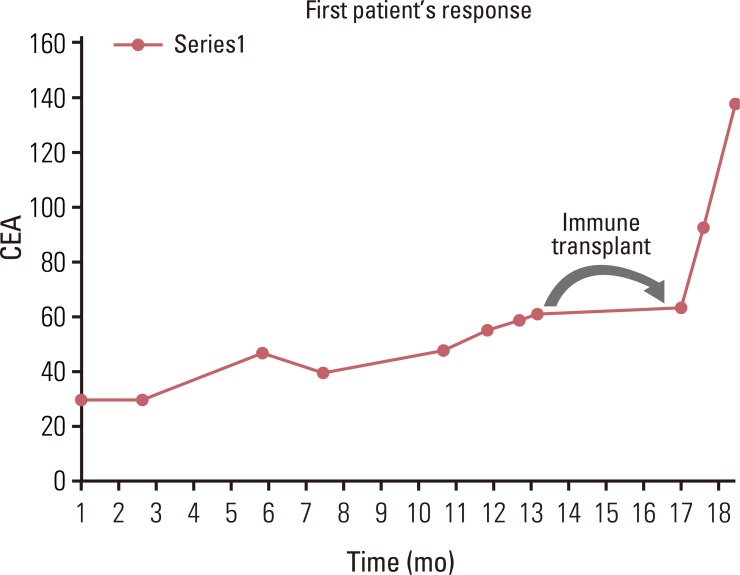
The first patient on this clinical trial was a 69-year-old female who had progressive colon cancer metastatic to the lungs and bone, progressive despite 5-fluorouracil/leucovorin and subsequently irinotecan. She had progressive right-sided chest pain attributable to metastasis, requiring opioid analgesic for pain control. Her other medical problems included chronic obstructive pulmonary disease; of note, she did continue to smoke. Her donor was an HLA identical match, and thus a strong immune response was not expected. The donor was able to provide 5×107 mononuclear cells/kg weekly for 8 of the 10 planned weeks. These were irradiated with 2,500 cGy before infusion into the patient. Treatment was held due to IL-2 toxicity two weeks, such that she received a total of 6 infusions. The IL-2 toxicity included a grade 2 left sided pleuritic chest pain in the area of metastatic disease in the chest. This had an inflammatory character and seemed to decrease as the treatment progressed over several weeks, along with the decreased doses of IL-2 for toxicity. She was unable to tolerate more than 1.8×109 units/kg of the IL-2 along with the mononuclear cell infusions. Her cancer-related pain in the right chest resolved after the first infusion but returned slightly after a delay in her treatment. Her tumor markers paralleled her clinical response and plateaued while on therapy and then rose after her treatment was completed (Fig. 2). Her disease progressed rapidly after completion of the protocol treatment regimen, with recurrence of the original right-sided chest pain. She was enrolled in hospice care 6 months after starting treatment. No GVHD was seen in this patient.
3. Patient 2
The second patient was a 26-year-old male with recurrent clear cell sarcoma of the left rib metastatic to the chest cavity with right pleural, chest wall, and mediastinal disease. Following multiple surgical interventions, combination chemotherapy including cyclophosphamide, adriamycin, vincristine alternating with ifosfamide and etoposide, followed by second-line carboplatin and gemcitabine (as well as three courses of palliative radiation therapy to the chest), he was not considered a candidate for an National Cancer Institute-sponsored bone marrow transplant protocol. Thus, the patient was enrolled in the current protocol. At the time of treatment initiation, the patient was home-bound with severe chest wall pain, requiring combination long- and short-acting opioids, with dyspnea requiring home oxygen, as well as fatigue and nausea. Both donors were haploidentical with identical blood type. He experienced an early response to therapy, with complete resolution of his pain, resolution of dyspnea (including no longer requiring supplemental oxygen), and he was able to return to work. His major toxicities were muscle inflammation (for which he took non-steroidal and mild pain medications) and an inflammatory skin reaction at the IL-2 injection site, appearing identical to a classic delayed-type hypersensitivity (DTH) reaction, which paralleled his response. The muscle aches and pains replaced his cancer pain but he required less pain medications and improved with reductions in the IL-2 dose. All this changed dramatically when was involved in an accident and required surgery. His disease rapidly progressed with a return in his chest pain, difficulty breathing, nausea and fatigue. He never improved after this but rapidly deteriorated clinically. Imaging showed dramatic progression as well. The patient elected to continue on study, which allowed progression because of the delayed nature of immunotherapy. He continued to progress however and expired after the last mononuclear cell infusion. He exhibited no GVHD throughout his treatment course. A graph of the patient's performance status vs. his donor infusions suggests that the accident may have affected his response (Fig. 3).
4. Patient 3
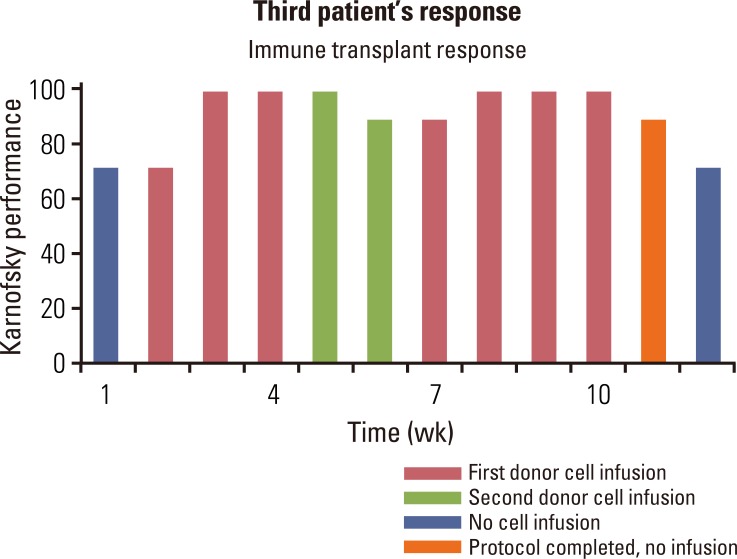
The third patient was a 45-year-old female with metastatic small-cell lung cancer who had undergone primary concurrent chemoradiotherapy for then-limited-stage disease, but experienced subsequent metastatic recurrence. Thereafter, she underwent salvage cisplatin/irinotecan with partial response; however, following this, she had rapid progression including bulky retroperitoneal adenopathy, with resultant back and flank pain. Following enrollment in the current study, two haploidentical related donors with identical blood types were identified. After the first three infusions (alternating donors), her back pain resolved completely and quality of life significantly improved, and she was thus able to resume her usual activities. Of note, following the third infusion she developed large DTH-like lesions at her IL-2 site injections (Fig. 4), and thereafter her dose of IL-2 was reduced by protocol design due to adverse effects (confluent erythematous rash, grade 2 muscle aches, decreased appetite, and decreased quality of life). Interestingly, the pain related to her cancer resolved after her first donor infusions, returned following second donor infusion, then resolved again following the third infusion (first donor). Thereafter, the first donor became her primary donor for the remainder of the study. After 10 cycles she was restaged and showed no progression in the retroperitoneal nodes, but progression in her adrenal gland for a mixed response. Her quality of life changes on study, associated with her donor infusions, are depicted in Fig. 5. Thereafter, she progressed rapidly in the retroperitoneal nodes, with associated recurrence of back pain. Further salvage chemotherapy was not effective until she received an irradiated blood transfusion from her first donor and her back pain resolved. Subsequently, she developed metastatic disease to the brain, and elected to proceed with hospice, 14 months after starting protocol therapy. She did not demonstrate any signs or symptoms of GVHD at any time during or after her immune transplant.
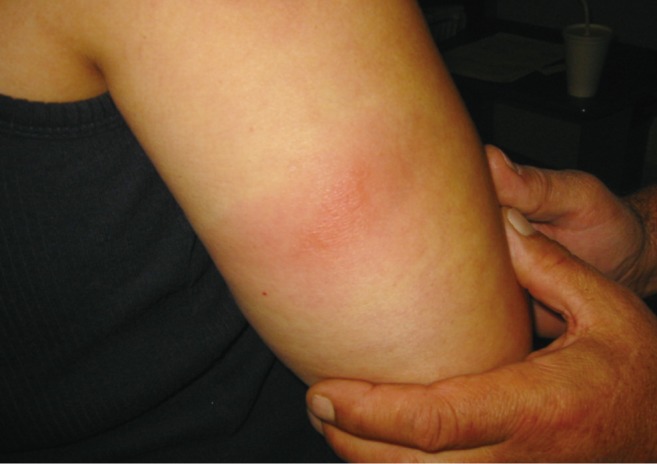
A typical skin reaction at the interleukin-2 injection site for those patients receiving haploidentical mononuclear cells.
A summary of the study patients' response and survival is given in Table 1.
5. Donors
The first donor could donate the protocol amount regularly and some of cells had to be discarded, however, all subsequent donors had difficulty providing sufficient concentration of mononuclear cells. The actual donor collection data for each patient is presented in Table 2. The maximum dose of mononuclear cells had been reached and no further increases were practical.
Discussion
The mechanism for the anti-tumor effect from the temporary engraftment of donor lymphocytes is currently unknown. It does not appear to be due to a simple allogeneic response by the donor mononuclear cells, but seems to be more prominent in haploidentical donor, non-myeloablative transplant regimens [11,12]. Animal models of this phenomenon suggest an interaction between the donor and host immune systems as part of the explanation. One model treated mice having established hematologic and solid tumors with cyclophosphamide followed by donor haploidentical or non-identical allogeneic mononuclear cell infusion [14]. These donor mononuclear cells also were all rejected in this model, yet they induced tumor regression and markedly improved survival in several different mice strains having established lymphoma, renal cell cancer and melanoma. The results were most consistent with the donor CD4 T cells providing help to host APCs, which in turn activated host CD8 T cells against the tumor. In addition, some of the anti-tumor effect appears to be directly from the donor lymphocytes, such as the donor CD8 T cells or NK cells.
However, breaking tolerance to cancer by the addition of donor mononuclear cells carries the risk of GVHD [15]. Therefore the concept of using irradiated mononuclear cells to break tolerance and stimulate an anti-tumor response without the risk of GVHD has been attempted. This treatment has been successful in animal models. In a murine model, haploidentical mononuclear cells irradiated with 750 cGy and activated with IL-2 were effective against murine breast cancer, however multiple doses were thought to be required for a sustained response in this system [16]. This strategy is also successful in human studies. Multiple doses of donor haploidentical donor mononuclear cells irradiated with 2,500 cGy have been given to patients with metastatic renal cell carcinoma [17]. Stable disease or partial response was seen in this study, and no GVHD was noted. The regimen involved 1-3 infusions of 0.9-3.6×108 CD3+ cells/kg every 8 weeks. Responses in this study were delayed for several months after initiation of therapy. The authors postulated that the donor irradiated mononuclear cells (e.g., NK cells, T cells, or macrophages) were altering the patient's immunity resulting in breaking tolerance to the tumor and developing a delayed patient anti-tumor immune response. Potential modifications to improve response include less radiotherapy dose or increased concentration of mononuclear cells (when possible) [18]. In another study, using lower doses of radiation, donor mononuclear cells irradiated with 750 cGy were infused into patients with stem cell transplant-refractory hematologic malignancies [13]. In this approach, approximately six infusions of irradiated 1×107 T cells/kg were infused over a two-week period. No GVHD was seen, yet two patients with leukemia attained a complete remission and one patient with lymphoma had a partial response for a response rate of 25%. In this study, patients who relapsed were able to be salvaged with non-irradiated mononuclear cells, again indicating a decreased effect of irradiation on mononuclear cell activity.
In addition, standard chemotherapy, under certain circumstances, can favorably affect the anti-tumor immune response, in order to help break the tolerance to tumor cells (rather than immunosuppression). This was shown in a recent study similar to our own, in which immunomodulatory agents were administered to patients with metastatic colon cancer following standard chemotherapy [18]. Chemotherapy consisted of gemcitabine plus oxaliplatin, 5-fluorouracil and folinic acid followed by GM-CSF and then low dose IL-2. This regimen, called the "GOLFFIG-1," tumors-pecific cytotoxic T cell frequency, reduced the regulatory T cells, restored the CD4/CD8 ratio and improved the median time to progression of 12.5 months in heavily pretreated patients.
Similarly, in an animal model, on which our study is based [19], mice were given lethal doses of leukemia and subsequently treated with etoposide and cyclosporine. Approximately 20% of these mice became long-term survivors who are immune to further leukemia challenges. These mice develop cytotoxic T cells against their leukemia, which has specific activity against the leukemia and not other leukemia cells or normal hematopoetic cells. These cytotoxic T cells can be expanded in the laboratory with tumor antigen from irradiated tumor cells, irradiated feeder spleen cells providing the antigen processing and presenting and T helper cell function, and T cell growth factor similar to IL-2. However, expanding these cytotoxic T cells in the laboratory is a labor-intensive and expensive process requiring considerable time, materials and manipulation. Therefore, in this pilot study, we attempted to expand the cytotoxic T cells in the patients themselves rather than in the laboratory. Instead of irradiated tumor cells as in the laboratory method, we chose to give chemotherapy followed by GM-CSF to increase the antigen processing and presenting of tumor antigens, equivalently to the GOLFFIG-1 chemo-immunotherapy above. Similar to the laboratory method and the GOLFFIG-1 chemo-immunotherapy, we chose to give IL-2 as a T cells growth factor. Instead of irradiated feeder cells as in the laboratory methold, we chose to use irradiated donor mononuclear cells to stimulate the patient's tumor antigen processing and presenting cells and helper cell activity and act as cytotoxic T cells and NK cells against the tumor, i.e., an immune transplant. We hoped that the addition of irradiated mononuclear cells would help reverse the tolerance against the cancer similar to the studies with irradiated haploidentical mononuclear cells and metastatic renal cell cancer. However, in this pilot study we added chemotherapy and immune modulating therapy to obtain a greater response.
This pilot study demonstrated the feasibility of this regimen in a community hospital setting, involving cooperation between four departments. Despite the fact that freezing facilities were not available, donor mononuclear cells were able to be collected weekly for all three patients, missing only 3 donations out of 30. However, this was challenging to accomplish and required scheduling and negotiating with substitute donors for each patient to the point that we recommend future studies employ frozen donor mononuclear cells instead of freshly donated monouclear cells. An average large single donor apheresis collection without GM-CSF stimulation is approximately 2×107/kg monouclear cells, yielding approximately 2×109mononuclear cells/kg. This is in the range of what we were able to accomplish with weekly infusions, and what is typically given in similar cellular therapies [13].
Despite enrollment of suboptimal candidates for immunotherapy (i.e., large volume of polychemorefractory disease in patients with compromised performance status), all three patients exhibited signals of activity. The first two patients were treated with mononuclear cells irradiated with 2,500 cGy before infusion. The first patient with advanced colon cancer received mononuclear cells, which were a full HLA match and not expected to produce a strong immune response. However, she had stabilization of her disease while on study and progressed rapidly after completion of the treatment. The second patient received donor cells that were HLA haploidentical and expected to produce a stronger immune response. In fact, he had a dramatic clinical response with resolution of his cancer related pain and a return to a normal lifestyle. In parallel with his clinical response, he developed large thick skin lesions at the injection sites similar to classic DTH reactions. His strongest DTH-like reactions occurred after the first donor infusions. However, after an accident requiring surgery, his disease rapidly progressed, his pain returned and his quality of life deteriorated dramatically. What caused the reversal in response in this patient is unclear; trauma and surgery are known to induce immune suppression [20], a decrease in lymphocyte activation (as measured by CD71 and CD 25, antigen-specific helper activity) [21], NK cell activity [21], and DTH response [22]. In fact, his DTH-like reaction decreased dramatically after his accident, indicating a decrease in his immune response along with his worsening clinical symptoms. This would suggest a delicate balance between anti-tumor activation and tolerance and the need for immune modulation for optimal response.
To our knowledge, this pilot study was the first to employ irradiated mononuclear cells immediately failing standard chemotherapy. It is noteworthy that no patient demonstrated signs of GVHD, and the maximum number of mononuclear cells appears to have been reached. Therefore, another mode by which to increase efficacy may involve reducing the dose of radiotherapy. The optimal dose of irradiation has not been established, but HLA-matched donor lymphocyte infusions irradiated with 750 cGy have been given to patients with chronic myelogenous leukemia and lymphoma at comparable cell doses without GVHD [13]. Our best response occurred with mononuclear cells irradiated with 750 cGy (patient 3), leaving the door open for future trials with lower doses of irradiated mononuclear cells after standard chemotherapy. Another option for improving response may be to increase the duration of "maintenance" infusions. In support of this approach, Patient 3 demonstrated objective (radiographic) and subjective (decrease in cancer-related pain scoring) response after fifth line salvage chemotherapy, when infused with her first donor's irradiated mononuclear cells.
After this protocol was initiated, it became evident that IL-2 actually activates two opposing pathways. Not only does it stimulate the proliferation and function of CD4+ and CD8+ lymphocytes against the cancer but it also induces activation induced cell death of CD4+ T cells and the survival and function of T regulatory cells which protect against autoimmunity and also suppress the anti-tumor response [23]. Therefore, giving constant IL-2 along with mononuclear cells, as was done here, may not be the optimal way to deliver this therapy. In fact, the best response for the three patients was after several mononuclear cell infusions following the chemotherapy. Therefore, continuing the IL-2 (as in this protocol) may result in further immune suppression rather than activating the immune response against the cancer. Giving mononuclear cells for several cycles after sequential chemotherapy may be the optimal way to deliver this cellular therapy.
This immune transplant design lends itself to further development with immune modulating agents other than IL-2, to further boast the anti-tumor response and tip the balance of the immune system in favor of the patient. Recently, irradiated HLA partially matched donor mononuclear cells irradiated with 2,500 cGy were given along with high doses of thalidomide as an immune modulating agent to a patient with advanced renal cancer, resulting in a complete remission [24]. Other immune modulating agents could be combined with this form of therapy as well; for example, anti-cytotoxic T-lymphocyte antigen-4 (anti-CTLA-4) antibodies that release the braking action of CTLA-4 on the immune system and have single agent activity against metastatic melanoma [25]. By measuring the anti-tumor immune response, such as with tetramer and enzyme-linked immunosorbent spot (ELISPOT) analysis, we may be able to optimize this form of immune therapy and better break tolerance to the patient's cancer. We may even be able to tailor this therapy for each individual cancer and patient.
Conclusion
In summary, irradiated mononuclear cells after standard chemotherapy is a feasible cellular therapy to deliver in a community hospital setting and shows promise to help reverse the patient's tolerance toward the tumor. The optimal schema for this therapy has not been established, including but not limited to the dose of irradiation used to prevent GVHD and yet maintain effectiveness, the best immune modulating agents to use, the cancers most sensitive to this treatment, the optimal chemotherapy to use with this treatment which may vary with disease, and whether mononuclear cells after sequential chemotherapy are more effective.
Acknowledgments
MedCenter One Foundation provided funding to support this investigation.
Notes
Conflicts of interest relevant to this article was not reported.