Interval between Surgery and Radiation Therapy Is an Important Prognostic Factor in Treatment of Rectal Cancer
Article information
Abstract
Purpose
The purpose of this study is to evaluate survival and prognostic factors for rectal cancer, including interval between surgery and radiation therapy after surgery, radiation therapy, and chemotherapy.
Materials and Methods
We conducted a retrospective study of 153 patients with rectal cancer who were treated with surgery, radiotherapy with/without chemotherapy at Keimyung University Dongsan Medical Center from January, 1988 to December, 2005. The study included 89 males and 64 females, with a median age of 56 years (range, 23 to 81 years). Tumor, node and metastasis (TNM) was I in 23 patients, II in 39, and III in 91. Radiation therapy was performed on pelvic fields using a median dose of 54 Gy five days per week, 1.8 Gy once per day. Ninety two patients were treated with radiotherapy, 43 with concurrent chemo-radiation therapy and 18 with sequential therapy after surgery. The median follow-up period was 52 months (range, 4 to 272 months). The interval between surgery and radiation was 1-25 weeks (median, 5 weeks).
Results
Two-year and five-year overall survival rate was 64.7% and 46.4%, respectively. Two-year and five-year disease-free-survival (DFS) rate was 58.6% and 43.1%, respectively. Median DFS was 39 months. Loco-regional failure was evident in 10.5% of patients, 8.4% had distant metastasis, and 9.2% had both. In multivariate analysis, TNM stage and interval between surgery and radiation therapy (≤5 weeks vs. >5 weeks; 95% confidence interval, 1.276 to 2.877; hazard ratio, 1.916; p=0.002) were significant prognostic factors of DFS.
Conclusion
Survival rates for rectal cancer after surgery, chemotherapy, and radiation therapy were similar to those reported in previous studies. Starting radiation therapy as soon as possible after surgery, especially within the first five weeks after surgery, is suggested.
Introduction
The problem of an unacceptably high local recurrence after surgery in patients with rectal cancer has led to conduct of many studies exploring the potential benefit of postoperative adjuvant therapy [1]. One of the advantages of postoperative radiation is the ability to provide selective treatment for patients at high risk of local failure on the basis of pathologic stage. Disadvantages include a potentially hypoxic postsurgical bed, which makes radiation less effective, and the potential for more severe complications due to increased small bowel volume in the radiation field, and a larger treatment volume, especially if the patient undergoes an abdominoperineal resection (APR) and the perineal scar needs to be covered [2]. Postoperative radiation with or without chemotherapy has been investigated in several large trials [3,4]. In general, surgery alone has resulted in a 25% local failure rate and 40-50% overall survival (OS) for T3/T4 or node positive patients, while radiation with the addition of chemotherapy has yielded a lower local failure rate of 10-15% and a higher OS rate up to 50-60%.
Based on the results of several randomized studies [4-6], the National Institutes of Health Consensus Conference recommended combined use of radiation and chemotherapy as a more effective treatment than postoperative radiation alone, with a greater potential for improved survival [7]. One study reported on protracted infusion of fluorouracil during pelvic irradiation, which resulted in improvement of the effect of postoperative radiation therapy (RT) in patients with high-risk rectal cancer [8].
The time interval from surgery to radiotherapy may have an effect on the local recurrence rate in patients with endometrial cancer not receiving chemotherapy and every possible effort should be made to start radiotherapy within nine weeks [9]. However, a consensus on the optimum interval between surgery and radiation in postoperative RT of rectal cancer has yet to be reached [2].
We conducted this study retrospectively in order to evaluate long-term survival and prognostic factors, including interval between surgery and radiation in patients with rectal cancer treated with surgery followed by radiotherapy with or without chemotherapy.
Materials and Methods
We conducted a retrospective study of 188 patients with rectal cancer treated with surgery, postoperative RT with or without chemotherapy at Keimyung University Dongsan Medical Center from January, 1988 to December, 2005. Thirty-five patients who did not receive regular follow-up after completion of treatment were excluded from this study. A total of 153 patients were analyzed. Patients underwent physical examination, complete blood cell count, blood chemistry, chest X-ray, pelvic computed tomography (CT), colonoscopy, and biopsy to confirm the presence of disease.
Characteristics of patients are shown in Table 1. The study included 89 males and 64 female patients ranging in age from 23-81 years (median, 56 years). According to the American Joint Committee on Cancer (AJCC) tumor, node, and metastasis (TNM) 7th edition staging method, there were 23 patients with stage I, 35 with stage IIA, four with IIB, one with IIIA, 39 with stage IIIB, and 51 with IIIC. All patients had pathologically proven adenocarcinoma. The degree of differentiation was well differentiated in 16 patients, moderately differentiated in 86 patients, poorly differentiated in six patients, and unknown in 45 patients. Thirty six patients (23.5%) had perineural invasion (PNI), 101 patients (66%) had lymphovascular invasion (LVI), and 12 patients (7.9%) had a positive urgical margin in the surgical specimen. The interval between surgery and RT was 1-25 weeks (median, 5 weeks).
Following surgery, 92 patients (60.1%) underwent RT alone, 43 (28.1%) underwent concurrent chemoradiation, and 18 (11.8%) underwent sequential radio-chemotherapy. Methods of surgery included local excision (n=21), low anterior resection (n=73), and APR in 59 patients. Irradiation of most patients was performed with the patient in the prone position. Patients received 45 Gy of radiation with 6/10/15MV photons for five days per week and 1.8 Gy once per day; whole pelvis with three or four fields (right, left, and posterior-anterior, with or without anterior-posterior). In patients with gross tumor or positive surgical margin after surgery, radiation dose was boosted by field reduction following whole pelvic RT, and the total dose was 43.2-63 Gy (median, 54 Gy). Chemotherapy usually included 5-fluorouracil (5-FU)-based infusion therapy, and, typically, six cycles of continuous infusion, 5-FU/cisplatin was given. Delivery of 5-FU (1,000 mg/m2) with 5% dextrose in 500 mL water was started as a continuous intravenous infusion on days 1-4 with a three-week interval (Table 2). Follow-up duration was 4-272 months with a median of 52 months. Time to local failure and distant metastases was analyzed starting from the day of surgery after diagnosis.
The Kaplan-Meier method was used to estimate the rate of OS and disease-free survival (DFS). A log-rank test was used in performance of univariate analysis for evaluation of possible prognostic factors associated with OS and DFS. Factors found to influence survival in univariate analysis were then analyzed by Cox proportional hazard regression analysis. SPSS ver. 17.0 (SPSS Inc., Chicago, IL) was used in performance of statistical analysis.
Results
1. Survival and prognostic factors
Five-year overall survival rates (5YOS) and five-year disease free survival rates (5YDFS) were 46.4% and 43.1%, respectively (Fig. 1).

Overall and disease free survival curves. YOS, year overall survival; YDFS, year disease free survival.
We analyzed several factors that may affect OS and DFS, including patient age, gender, stage, pathologic differentiation, PNI, LVI, surgical margin status, treatment modality, and interval between surgery and radiation. In univariate analysis for identification of potential prognostic factors related to OS and DFS, TNM staging, PNI, LVI, and interval between surgery and radiation were statistically significant and the treatment modality was marginally significant (Table 3). 5YDFS was 82.4% in stage I, 53.6% in stage II, and 28.6% in stage III. 5YDFS was 50.5% in patients for whom the interval between surgery and radiation was within five weeks, and 32.9% in those for whom the interval exceeded five weeks (Figs. 2 and 3). No statistical significance associated with patient age, gender, and pathologic differentiation was observed. 5YDFS was 47% in patients without PNI, 29.9% in those with PNI, 59.4% in patients without LVI, and 35.4% in those with LVI. In multivariate analysis for DFS, stage (95% confidence interval I vs. II, 1.289 to 10.266; hazard ratio [HR], 3.301; p=0.012; I vs. III, 3.163 to 27.226; HR, 9.281; p≤0.0001) and interval between surgery and radiation (95% confidence interval, 1.276 to 2.877; HR, 1.916; p=0.002) were found to be statistically significant (Table 4). Except for sex and treatment type, no statistically significant differences in patients' characteristics with regard to the interval between surgery and radiation were observed in the two groups (Table 5). The ≤5 week group included a larger number of male patients. The >5 week group included a larger number of patients treated with surgery and chemotherapy and subsequent radiotherapy. The data support initiation of RT as soon as possible after surgery, especially within the first five weeks.
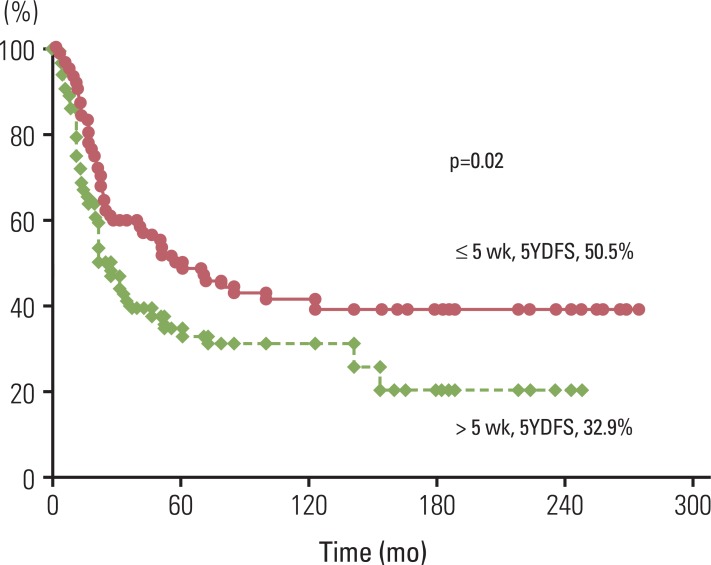
Disease free survival curves according to interval between surgery and radiotherapy. YDFS, year disease free survival.
2. Patterns of treatment failure and status
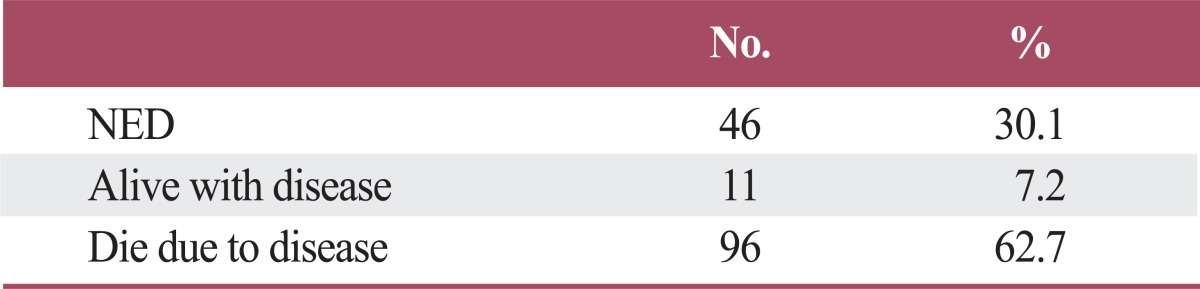
Sixteen patients (10.5%) had loco-regional failure, 13 patients (8.4%) had distant metastasis, and 14 patients (9.2%) had both loco-regional failure and distant metastasis (Table 6). In loco-regional failure, 26 patients showed recurrence in the tumor bed and four patients had nodal failure. Sites of distant metastasis included the liver in eight patients, lung in seven patients, bone in three patients, brain in one patient, and multiple organ failure in 8 patients. Patients' status was disease free in 46, alive with disease in 11, and 96 patients died due to rectal cancer (Table 7).
Sixteen patients had local recurrence only after postoperative radiotherapy. Local recurrences were almost always in organs of the pelvic cavity. Of the 10 patients with tumor bed recurrence, three patients underwent surgery and chemotherapy, two patients underwent surgery, one patient received chemotherapy, and three patients refused further treatment. The remaining patient with recurrence of levator ani muscle underwent mass excision and radiotherapy. Of the five patients with recurrence of multiple organs, one patient received adhesiolysis and chemotherapy. The other patients refused further treatment. Methods of operation included adhesiolysis, mass excision, or Miles' operation. Chemotherapeutic agents used included 5-FU, xeloda, irinotecan, and leucovorin. One patient with lymph node recurrence received chemotherapy and radiotherapy. The duration from the end of radiotherapy to recurrence was 7-81 months (median, 22 months). Median survival from diagnosis of local recurrence was 11 months. One patient with vaginal recurrence showed no evidence of disease after salvage treatments and is still alive.
Thirteen patients had distant metastasis only after postoperative radiotherapy. Distant metastases were almost in the liver, lung, and bone. Duration from the end of radiotherapy to distant metastasis was 0-120 months (median, 14.5 months). Specific sites included the liver (n=5), lung (n=3), multiple organs (n=3), and other sites, including para-aortic lymph node and peritoneal cavity (n=2). Treatment included chemotherapy for four patients, surgical excision of liver and lung metastasis for one patient, and chemotherapy and radiation for two patients. Four patients refused further treatment. Median survival from diagnosis of distant metastasis was 15 months.
Fourteen patients had both local recurrence and distant metastasis. Local recurrence was observed in the rectum, including the anastomosis site or pelvic lymph nodes and distant metastases, including liver, lung, brain, bone, abdominal wall, or peritoneum at 1-59 months (median, 10.5 months) after completion of radiotherapy. Eight patients underwent surgical resection of a local recurrent tumor, two patients underwent local excision, and six patients underwent a Miles' operation. Of the seven patients with liver metastases, one patient underwent surgical resection and one patient underwent trans-arterial chemo-embolization. Four patients received palliative chemotherapy, including oral capecitabine, doxifluridine, or intravenous 5-FU. The other two patients refused further treatment. Median survival from diagnosis of recurrent disease was eight months.
Discussion
Recently, because of the capability of down staging, preservation of anal sphincter function, and survival benefit following improvement of local control rate, preoperative concurrent chemo-radiotherapy and surgery have been recommended as standard treatment for patients with locally advanced rectal cancer. However, the problem of high local recurrence after surgery has led to exploration of the potential benefit of postoperative RT [1,3,10]. In early stage rectal cancer, local excision alone yields a 90% survival rate; however, adjuvant RT due to local failure is required in 9-20% of cases [11,12]. Also, in patients with T3, T4, or node positive, postoperative chemo-radiotherapy show a decrease above 10% of local failure. Two National Surgical Adjuvant Breast Bowel Project (NSABP) trials concluded that while postoperative radiation treatment did not appear to result in improvement of OS, there was an improvement in local control [5,6]. Results of two trials, the Gastrointestinal Tumor Study Group (GITSG) and North Central Cancer Treatment Group (NCCTG) studies, did show an improvement in survival [4,13]. According to findings of the GITSG trial, postoperative chemotherapy and RT resulted in improvement of OS to 54% vs. 27%, and prolonged time to recurrence and a decreased recurrence rate of 33% vs. 55% with observation after surgery [13]. Findings of the Mayo-NCCTG trial indicated that combined postoperative chemotherapy and RT resulted in reduced recurrence by 34%, local recurrence by 46%, distant metastasis by 37%, cancer deaths by 36%, and overall deaths by 29% [4]. Based on the results of these studies, the National Institutes of Health Consensus Conference recommended combined use of radiation and chemotherapy as a more effective treatment than postoperative radiation alone [7]. The intergroup 0114 study, which compared different chemotherapy regimens with radiation, reported no difference in OS or DFS among four groups [14].
We performed this retrospective study in order to evaluate long-term survival and prognostic factors, including interval between surgery and radiation in rectal cancer patients treated with surgery and postoperative radiotherapy with or without chemotherapy. Survival rates were satisfactory, although they could not be exactly compared with those reported in other trials. According to findings of a meta-analysis of randomized studies, OS was only marginally better in patients who underwent radiotherapy, compared with those who did not receive radiotherapy, with 45.0% vs. 42.1% alive at five years, the yearly death rate was 5.4% (SE 2.9; 95% confidence interval, 0 to 11%) lower in patients who had undergone radiotherapy than in those who had none; the reductions did not differ significantly between patients who had undergone preoperative radiotherapy and those who had undergone postoperative radiotherapy [3]. However, since the introduction of total mesorectal excision (TME), local control in patients undergoing rectal cancer surgery has shown substantial improvement [15]. In one study, no added benefit was observed in terms of local recurrence or OS in stage IIA rectal cancer patients receiving radiotherapy following TME with adjuvant CT [15]. TME has been performed since 2002 in our hospital, and only 26 patients were enrolled in this study. 5YOS and 5YDFS according to treatment type were 46.6% and 43.4% in surgery and postoperative RT, 53.5% and 51.1% in surgery and postoperative concurrent chemo-radiation (CCRT), and 27.8% and 22.2% in surgery and chemotherapy and subsequent radiation. Postoperative CCRT yielded better survival compared with postoperative radiation or postoperative chemotherapy and subsequent radiation. Survival rates for the postoperative CCRT or postoperative radiation group showed comparable results with those reported in randomized trials [13,14]. However, patients treated with surgery and chemotherapy and subsequent radiation showed relatively poor survival. One study reported an association of postoperative RT with improved survival for patients who underwent curative resection for treatment of pancreatic, gastric, and rectal malignancies, and significant differences were observed for this effect according to stage of disease, with more advanced cases generally experiencing a greater benefit with RT [16]. The interval between surgery and radiation, which was a significant prognostic factor in DFS, was 3-23 weeks (median, 13 weeks). In addition, the treatment modality was not a prognostic factor in DFS. Delivery of postoperative radiation in rectal cancer patients with risk factors for recurrence as soon as possible appears to be beneficial.
Several factors have been shown to have a significant impact on tumor behavior. TNM stage clearly remains the dominant determinant of survival [17]. According to results reported from one study, lymph node involvement, tumor extension beyond the bowel wall, and high histologic grade were prognostic factors showing an independent association with poorer survival and increasing distant failure. Use of chemotherapy was associated with a significant improvement in survival and a decrease in distant failure. No single factor showed a significant association with local failure. Adequate perineal coverage after combined APR yielded significantly fewer perineal failures [18]. In univariate analysis, LVI has been shown to have a negative impact on survival [19,20]. In univariate analysis for identification of potential prognostic factors related to OS and DFS, TNM staging, PNI, LVI, and interval between surgery and radiation were statistically significant and the treatment modality was marginally significant. However, in multivariate analysis, TNM staging and interval between surgery and radiation were statistically significant. 5YDFS was 50.5% in patients having a five-week interval between surgery and radiation and 32.9% when the interval exceeded five weeks. Despite the lack of consensus with regard to the optimum time for start of postoperative radiation, it is recommended that treatment should begin 3-6 weeks after surgery [2]. This recommendation is supported by the present multivariate analysis for DFS.
Knowledge of the optimum time to start postoperative radiation is important. With this aim, we attempted to determine differences in patients' distribution in the two groups with regard to the interval between surgery and radiation (≤ 5 weeks vs. >5 weeks). Statistically significant differences in patients' characteristics were evident in the two factors. In the group having an interval of ≤5 weeks, there were more male patients. In the group having an interval of >5 weeks, there were more patients who underwent treatment with chemotherapy and subsequently with radiotherapy than those treated with postoperative RT or CCRT (Table 5). If postoperative radiation and chemotherapy are needed in rectal cancer, postoperative CCRT or postoperative radiation and sequential chemotherapy may well be warranted. Also, RT should be initiated as soon as possible after surgery, especially within the five-week interval between surgery and radiation. Several studies have reported that an interval between chemo-radiation and surgery of ≥7-8 weeks is safe and is associated with a higher rate of pathologic complete response as well as decreased local recurrence and improved DFS [21,22]. These studies involved preoperative RT and this enhanced effect of a prolonged time interval on tumor response may be supported by the fact that radiation induced necrosis is a time-dependent phenomenon. In postoperative RT, the effect of the interval between surgery and radiation would be different. The rationale for postoperative radiation is based on the fact that elimination of subclinical foci of tumor cells in the tumor bed, including lymph node metastasis, is possible. However theoretic and experimental evidence suggests that the radiation effect may be impaired by vascular changes occurring in the tumor bed after surgery [23]. Therefore, by delivery of higher doses to the volume of high risk or known residual disease, better tumor control may be achieved with postoperative radiation than with preoperative irradiation. However, because this study was not prospectively designed, the time interval between surgery and radiation with or without chemotherapy was decided according to patients' condition and economic status and the individual surgeon's preference. Traditionally, our general approach has been to wait 4-6 weeks after surgery; however, there were often several factors, including patient morbidity, patients' cooperation, financial problems, and chemotherapy. Data from this study support the start of RT as soon as possible after surgery, especially within the first five weeks.
Conclusion
The present survival rates for patients with rectal cancer after surgery, chemotherapy, and RT are similar to those reported in previous studies. Stage and interval between surgery and RT were statistically significant prognostic factors in DFS after postoperative radiation in patients with rectal cancer. Starting RT as soon as possible after surgery, especially within the first five weeks after surgery, is suggested.
Notes
Conflict of interest relevant to this article was not reported.