A Case Report of Paraneoplastic Pemphigus Associated with Esophageal Squamous Cell Carcinoma
Article information
Abstract
Paraneoplastic pemphigus is an autoimmune blistering and erosive mucocutaneous syndrome associated with underlying neoplasm. It is primarily associated with lymphoproliferative disorders, and uncommonly with malignancies of epithelial origin. We report on a case of a 68-year-old male who presented with whole body bullous and erosive skin lesions. Findings on upper gastrointestinal endoscopy and skin biopsy revealed esophageal squamous cell carcinoma and paraneoplastic pemphigus. Palliative chemotherapy and systemic glucocorticoid were started, however, the patient died of overwhelming sepsis on the ninth day of chemotherapy. This case demonstrates that paraneoplastic pemphigus can occur in esophageal squamous cell carcinoma and could be a cause of morbidity.
Introduction
Paraneoplastic pemphigus (PNP), an acantholytic mucocutaneous syndrome associated with malignancy, is characterized by painful mucosal erosions, ulcerations, and polymorphous skin lesions that progress to blistering eruptions on the trunk and extremities [1]. PNP is thought to arise from antibodies directed against tumor antigens that exhibit cross-reactivity against various epidermal proteins [2]. Autoantibodies bind to epidermal proteins responsible for adhesion, resulting in skin detachment [3].
PNP is most frequently associated with hematologic malignancy or disorders such as non-Hodgkin's lymphoma, chronic lymphocytic leukemia, and Castleman's disease [2], comprising more than 80% of PNP associated neoplasm. However, various non-hematologic solid tumors, including carcinoma, sarcoma, and melanoma have also been reported to show an association with PNP. As for carcinomas, cases of adenocarcinoma of the pancreas, gastrointestinal tract, breast, squamous cell carcinoma of the tongue, vagina, hepatocellular carcinoma, and renal cell carcinoma have been reported in association with PNP [4-7], however, their occurrence is very rare; only 13 cases among 163 PNP cases reported during a period of 13 years were carcinomas [5]. Although non-hematologic malignancies are infrequently associated with PNP, they should be considered in evaluation of a patient presenting with pemphigus. Here, we report on a case of a patient with esophageal squamous cell carcinoma who presented with PNP of the skin and showed rapid deterioration despite steroid treatment and palliative chemotherapy.
Case Report
A 68-year-old male presented to the emergency room (ER) with progressive bullous and erosive skin lesions involving the whole body. Two weeks earlier, the patient had visited the ambulatory clinic with a painful oral ulcer. At that time, bilateral enlarged supraclavicular lymph nodes were observed on physical examination, and fine needle aspiration of the lymph node revealed metastatic squamous cell carcinoma. The patient was diagnosed with diabetes 20 years ago and had been treated with diabetic nephropathy since eight years ago. He denied any recent changes in medication.
At presentation to the ER, he showed a blood pressure of 93/64 mmHg, a heart rate of 91/min, and a respiration rate of 20/min. Flaccid blisters, exfoliated skin, and crusted erosions involving eyelids, buccal mucosa, tongue, trunk, back, and extremities were observed (Fig. 1A-C). Laboratory tests showed anemia and thrombocytopenia: white blood count 5,170/mm3, hemoglobin 7.7 mg/dL, and platelets 426,000/mm3. He showed azotemia with blood urea nitrogen 52.3 mg/dL (normal, <22 mg/dL) and creatinine 2.98 mg/dL (normal, <1.3 mg/dL), which was due to already known diabetic nephropathy. Findings on computed tomography scan of the chest and positron emission tomography scans revealed multiple cervical and mediastinal lymph node enlargement with increased fluorodeoxyglucose uptake.
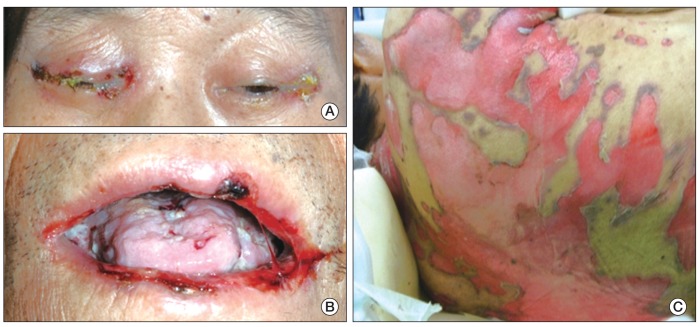
Clinical photographs. (A) The patient's eyelids showed crusted erosions. (B) Oral mucosa showed multiple ulcerative lesions and white exudates. (C) Flaccid blisters and exfoliated skin were observed on the patient's back.
To evaluate the primary site of metastatic squamous cell carcinoma, upper gastrointestinal endoscopy was performed; a deep ulcerative mass covered with yellow exudates was detected on the mid to distal esophagus. The biopsy showed moderately differentiated squamous cell carcinoma (Fig. 2A). Skin biopsy was also performed on the abdominal skin lesion and the histology showed suprabasal acantholysis with bullous cleft formation (Fig. 2B) and C3 deposits in the basal layer of mucosa, which was compatible with PNP. The patient was finally diagnosed with PNP associated with esophageal cancer of clinical stage IVB (American Joint Committee on Cancer, 7th edition).
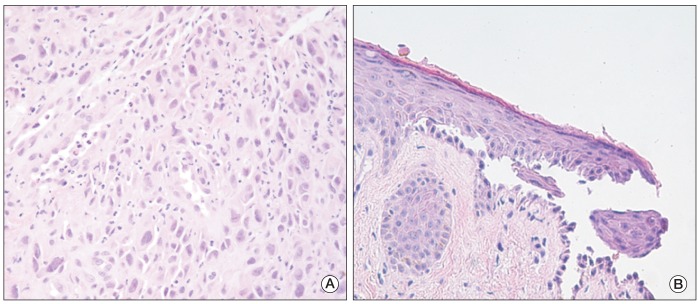
Histopathologic findings of esophageal mucosa and skin. (A) Squamous cell carcinoma with tumor cells showing variable nuclear size and sheet-like proliferation in the esophageal mucosa (H&E staining, ×400). (B) Acantholytic cells and bullous cleft formation were observed in the suprabasal layer of the skin (H&E staining, ×400).
The patient's skin lesion and general condition showed rapid aggravation, therefore, the patient was admitted to the intensive care unit for close observation and supportive care. Palliative combination chemotherapy with fluorouracil and carboplatin was started for control of the underlying esophageal cancer activity. We chose carboplatin instead of cisplatin because of the underlying diabetic nephropathy. Intravenous methylprednisolone 40 mg/day was also started simultaneously for treatment of pemphigus. Despite the concomitant chemotherapy and steroid treatment, the skin lesion worsened, with extensive skin defects, progressive exfoliation, and increased discharge.
On the sixth day of chemotherapy, neutropenic fever developed. Although broad spectrum antibiotics were started immediately, he expired due to subsequent overwhelming sepsis on the ninth day of chemotherapy.
Discussion
PNP is a life-threatening autoimmune disease characterized by cutaneous blister and erosions with mucosal involvement. PNP was first described as a distinct entity in 1990 [8], since then, more than 200 cases have been reported. It is mostly seen in association with lymphoproliferative disorders, and infrequently in some solid tumors, but is very rare in association with esophageal cancer.
The diagnosis of PNP is based on clinical features, histology, direct and indirect immunofluorescence (DIF and IIF), and immunoprecipitation tests.
Revised diagnostic criteria reported by Camisa and Helm [9] include major signs of polymorphic mucocutaneous eruption, concurrent internal neoplasia, and serum antibodies with a specific immunoprecipitation pattern and minor signs of histologic evidence of acantholysis, DIF showing intracellular and basement membrane staining, and IIF staining with rat bladder epithelium. Three major or two major and two minor signs are needed for diagnosis.
Recent studies have suggested that patients with PNP have characteristic autoantibodies against the plakin family proteins (e.g., envoplakin and periplakin) [10]. Antibodies against desmoglein 1 and 3, which are known to be target antigens of classic pemphigus vulgaris, were suggested to play some role in the initial stages of development of PNP [11]. More recently, due to the marked cellular inflammation in many cases and PNP cases without detectable autoantibodies, not only humoral immunity but the role of T lymphocytes has also been discussed [12].
Nearly all cases of PNP are accompanied with ulcerative oral or lip mucosal lesions, which is the first sign of PNP in nearly half of cases. Suspicion and awareness are required in order to recognize this rare disease when a patient presents with ulcerative oral lesions, although it can be due to other conditions, such as infection, reaction to drugs, or autoimmune diseases [5]. In the current patient, oral mucosal lesion was the initial chief complaint, however, the association of oral lesion and suspected malignancy was not recognized at his first presentation.
It has been suggested that PNP often shows resistance to treatment [9] with a high mortality rate of 75-80% [1]. The skin and mucosal eruptions cause significant morbidity, and are a frequent cause of death rather than the malignancy itself [9]. The treatment of PNP has not yet been well established. Most authors have suggested that early detection and removal of the primary tumor is essential for treatment of PNP, and systemic corticosteroid is regarded as the first line treatment [9]. The addition of steroid-sparing agents, such as azathioprine, cyclosporine A, or mycophenolate mofetil may reduce administration of steroid and thus limit side-effects. Immunoablative high-dose cyclophosphamide without stem cell rescue has been used in some cases, however, the benefits are still controversial [13]. Rituximab, the monoclonal antibody to CD20, has shown an association with improvement in a few patients [14]; however, others have failed to benefit from this agent [15]. Intravenous immunoglobulin (IVIG) has been used in some cases, and may be a worthwhile therapeutic adjunct for patients with PNP. Conduct of additional studies is necessary in order to determine the role of rituximab and IVIG in PNP.
Three cases of esophageal cancer associated PNP have been reported so far, two cases were reported before recognition of the disease entity in 1990 [4,6], and one English case was reported by Takahashi et al. [7] in which a patient was diagnosed with skin and esophageal PNP associated with esophageal squamous cell carcinoma in Japan and was treated successfully with curative resection of esophageal cancer followed by postoperative systemic steroid treatment. Changes in autoantibody titers were well correlated with disease activity. In contrast, the current patient had distant metastases; therefore, palliative chemotherapy was the only treatment option for control of the underlying malignancy. Immunocompromised state due to both chemotherapy induced neutropenia and high dose steroid administration, and extensive defects of skin barrier by pemphigus lesions made the patient extremely susceptible to infection and, eventually, he died of sepsis.
In conclusion, this case demonstrates that esophageal squamous cell carcinoma can be a rare cause of PNP, which could be associated with poor prognosis, as previously reported [1,9]. Early recognition of the disease and successful control of both underlying cancer and skin lesion is important. Infection control is the key component in treatment of PNP, especially when there are no other options for treatment of underlying malignancy but chemotherapy.
Notes
Conflict of interest relevant to this article was not reported.