Site-Specific Mutagenesis in Human Cells by Bulky Exocyclic Amino-Substituted Guanine and Adenine Derivatives
Article information
Abstract
Purpose
7-Bromomethylbenz[a]anthracene is a well-known mutagen and carcinogen. The aim of this study is to determine the mutagenic potency of its two major DNA adducts [N2-(benz[a]anthracen-7-ylmethyl)-2'-deoxyguanosine (b[a]a2G) and N6-(benz[a]anthracen-7-ylmethyl)-2'-deoxyadenosine (b[a]a6A)] and the simpler benzylated analogs [N2-benzyl-2'-deoxyguanosine (bn2G) and N6-benzyl-2'-deoxyadenosine (bn6A)] in Ad293 human cells and to compare to their mutagenicity in human cells and E. coli.
Materials and Methods
The shuttle vector pGP50 is capable of replicating in E. coli and human cells. Modified nucleotides were positioned in the plasmid pGP50 in a manner similar to pGP10 as described (8). Adenovirus transformed human embryonic kidney cells (line 293) were transfected with a shuttle vector containing an adduct. Two days later, the plasmids were recovered and treated with DpnI to remove unreplicated DNA. DH10B E. coli were transformed with the plasmids. Bacteria were cultured with the media containing X-gal, IPTG and ampicillin. Bacteria transformed by the plasmid with the adduct-induced mutation in the initiation codon of lacZ' form white colonies whereas bacteria transformed by the plasmid without mutation form blue colonies.
Results
In the human cell site-specific mutagenesis system, bn2G exhibited weak mutagenicity and bn6A was not mutagenic, although b[a]a2G or b[a]a6A produced 8% and 7% mutant colonies, respectively. At the site of the adduct, b[a]a2G induced the G→T transversion mutation while b[a]a6A produced the A→G transition mutation.
Conclusion
These data indicate that bulkier b[a]a2G and b[a]a6A exhibit significantly greater mutagenicity in human cells than in E. coli.
INTRODUCTION
Mutation has been recognized to initiate carcinogenic processes. The initial event in this multistage process is the covalent modification of cellular DNA by various reactive chemical carcinogens (1).
It has been difficult to characterize the mechanism of an individual carcinogen-DNA adduct altering and damaging the structure or coding properties of DNA since chemical carcinogens can react with multiple sites on DNA bases as well as with several DNA bases simultaneously. Thus, the chemical synthesis of DNA sequences and plasmids containing a single carcinogen-modified base at a predetermined sequence site has been used as a probe for the analysis of the structural and biological effects of defined carcinogen-modified bases (2,3).
The environmental pollutant 7-bromomethylbenz[a]anthracene (7-BrMeBA), an electrophile acting directly, is a well-known mutagen and carcinogen (4~7). N2-(benz[a] anthracen-7-ylmethyl)-2'-deoxyguanosine (b[a]a2G) and N6-(benz[a]anthracen-7-ylmethyl)-2'-deoxyadenosine (b[a]a6A) are the major deoxyribonucleosides in DNA modified by 7-BrMeBA. The site-specific mutagenicity of the major DNA adducts produced by 7-BrMeBA, i.e., b[a]a2G and b[a]a6A, when the modified base was incorporated into either a double-stranded or gapped plasmid vector was previously examined in E. coli (8). This study revealed that these bulky aralkylated adducts did not exhibit significant mutagenicity, which was unexpected.
Several studies showed the difference in the mutagenic potency of exocyclic DNA adducts between E. coli and mammalian cells, which indicates that the mutation frequency was influenced by host cells (9~11). These results led to the comparison of the mutagenic potency induced by b[a]a2G and b[a]a6A in E. coli and human cells and to examine the mutagenicity of simpler benzylated derivatives of guanine (N2-benzyl-2'-deoxyguanosine, bn2G) and adenine (N6-benzyl-2'-deoxyadenosine, bn6A) (12) to assess the effect of the size of the exocyclic amino group substituent on mutagenic potency.
Here, this study reports the site-specific mutagenesis induced by four aralkylated nucleosides b[a]a2G, b[a]a6A, bn2G, and bn6A (Fig. 1) in human embryonic kidney cells (line 293). In addition, this study compares the mutagenic potency of these four aralkylated nucleosides in human cells and E. coli.
MATERIALS AND METHODS
The restriction enzymes were obtained from New England Biolabs, Beverly, MA and were dissolved in the buffer supplied by the manufacturer unless specified. T4 polynucleotide kinase, T4 DNA ligase, uracil DNA glycosylase, X-gal, and IPTG were purchased from USP Corp., Cleveland, OH. All other enzymes and reagents used in this study were described previously (13). Adenovirus-transformed human embryonic kidney cell (Ad293) (14) was obtained from Otsuka Pharmaceutical, Rockville, MD. 2'-Deoxyguanosine and 2'-deoxyadenosine were purchased from Sigma Chemical Co., St. Louis, MO. Most other reagents and solvents were purchased from Aldrich Chemical Co., Milwaukee, WI. 7-Bromomethylbenz[a]anthracene was prepared as described previously (15). N2-benzyl-2'-deoxyguanosine, N6-benzyl-2'-Deoxyadenosine, N2-(benz[a]anthracen-7-ylmethyl)-2'-deoxyguanosine, N6-(benz[a]anthracen-7-ylmethyl)-2'-deoxyadenosine and these bulky aralkylated adducts containing 16-base oligodeoxyribonucleotides were prepared as described (12). Synthetic DNA sequences containing N2-aralkylated guanine and N6-aralkylated adenine nucleosides, 5'-d* (A A C A G C C A T A* T G* G C C C )-3' and a complementary 16-mer 5'-C U U G G G G C C A U A U G G C-3' were prepared by a DNA synthesizer (Applied Biosystems, Inc., Model 380B) and purified by polyacrylamide gel electrophoresis. All oligonucleotides were characterized by enzymatic digestion and followed by chromatographic analysis as described previously (12).
1) Preparation of adduct containing plasmids
A pair of oligonucleotides were incorporated into the human mutagenesis shuttle vectors pGP50 using the procedure described previously (Fig. 2) (13,16~18). In these constructions, vector DNA was digested with BspMI restriction enzyme to remove the insert DNA sequence shown in the box in Fig. 2. The major fragment produced by BspMI digestion was isolated and recircularized by ligating a pair of oligonucleotides as illustrated in Fig. 2 into the plasmid where the insert had been. Following the recircularization, covalently closed plasmids were isolated.
2) Mutagenesis experiments in E. coli
The mutagenic potency induced by four bulky aralkylated adducts in E. coli was determined as described previously (8,16).
3) Mutagenesis experiments in human cells
The human embryonic kidney cells (line 293, ATCC CRL 1573) were transfected with the shuttle vector containing control oligonucleotide or the adduct containing oligonucleotides (Fig. 3). Cells were incubated for 48 hours to allow the replication of the shuttle vector. Subsequently, plasmid DNA was recovered and treated with DpnI to remove unreplicated vectors. The recovered plasmids were used to transform the alpha complementing E. coli strain DH10B (19). E. coli thus transformed were cultured with the media containing X-gal and IPTG and blue or white colonies were scored. The procedures for the growth and transfection of human cells, the recovery and DpnI treatment of the vectors, and transformation and growth of E. coli have been described previously (19).
4) Sequencing of mutant plasmids
To characterize the adduct containing the lacZ' inactivating mutation and control plasmids, white colonies were isolated and subcultured. Plasmid DNA was isolated and the region surrounding the lacZ' initiation codon was sequenced using a dye terminator cycle sequencing kit (Perkin Elmer, Foster City, CA) and a DNA sequencer (Applied Biosystems 373A, Foster City, CA)
RESULTS
1) Mutagenicity in E. coli
The significant mutation frequency was not detected by aralkylated adducts [bn2G, bn6A, b[a]a2G, and b[a]a6A] in the E. coli site-specific mutagenesis system as reported previously (8) (Fig. 4).
2) Mutagenicity in human cells
The site-specific mutation induction of the major DNA adducts produced by the aralkylating agent 7-bromomethyl benz[a]anthracene [b[a]a2G and b[a]a6A] and the simpler benzylated analogs (bn2G and bn6A) in the adenovirus-transformed human embryonic kidney cells Ad293 were examined.
pGP50 is a shuttle vector capable of replicating in both E. coli and human cells. Modified nucleotides were positioned in the plasmid pGP50 in a manner similar to pGP10 as described (8,13). Adenovirus transformed human embryonic kidney cells (line 293) were transfected with the adduct containing the shuttle vector. Two days later, plasmids were recovered and treated with DpnI to remove unreplicated DNA. The plasmids were used to transform DH10B E. coli. DH10B E. coli bacteria were cultured with the media containing X-gal, IPTG and ampicillin. The site-specific mutagenesis induced by the bulky adducts was evaluated by the sectored colony assay system as previously described (13). Bacteria with the adduct-induced mutation in the initiation codon of lacZ' grow as white colonies. Bacteria without mutation grow as blue colonies.
In the human cell site-specific mutagenesis system, DH10B cells transformed with the plasmid derived from the b[a]a2G containing gapped vector that has been replicated in 293 cells produced 8% mutant colonies. Similarly, DH10B cells transformed with the plasmid derived from b[a]a6A produced 7% mutant colonies. In contrast, bn2G exhibited weak mutagenicity and bn6A was not mutagenic (Fig. 5).

Colony phenotypes resulting from aralkylated nucleosides in human cells. The difference in mutagenicities was analyzed by one-way analysis of variance (ANOVA), followed by Duncan's multiple range t-test. A significant difference in mutagenicities between aralkylated nucleosides and control is indicated by *P<0.05. The value represents the mean±SE of three expriments.
3) Mutations in human cells
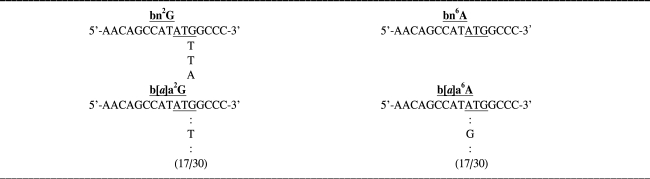
Thirty white colonies were grown following the tranformation with control plasmids or the plasmids harboring the adduct. Plasmid DNA was isolated and sequenced. In cells transformed with bn2G, two transversions, G-to-T, and one transition, G-to-A, were detected at the site of the adduct. The base change at the site of bn6A was not detected. In cells transformed with b[a]a2G seventeen transversions, G-to-T were detected at the site of the adduct. In cells transformed with b[a]a6A, on the other hand, 17 A-to-G transitions were detected at the site of the adduct (Table 1).
DISCUSSION
Recently, the site-specific mutagenesis in Escherichia coli by four bulky exocyclic amino-substituted guanines and adenines [bn2G, bn6A, b[a]a2G and b[a]a6A] incorporated site-specifically to the ATG initiation codon of the lacZ' gene in both double-stranded and gapped plasmids were reported (8). In this E. coli site-specific mutagenesis system, these aralkylated adducts did not exhibit the significant mutagenicity. The SOS induction also had no measurable effect on the mutagenicity of these adducts. The result that could not induce mutation in E. coli was totally unexpected since the environmental pollutant 7-bromomethylbenz[a]anthracene (7-BrMeBA) is a well-known mutagen and carcinogen. These data strongly suggest that the site-specific mutagenesis by these four bulky adducts should be examined in human cells. This study thus evaluated the site-specific mutagenicity of the major DNA adducts produced by the aralkylating agent 7-BrMeBA, [b[a]a2G and b[a]a6A] in human cells, when the modified base was incorporated into plasmid vectors and subsequently compared the mutagenic potency in E. coli and human cells. In addition, the effect of the size of the exocyclic amino group substituent on the mutagenic potency was examined by comparing the mutagenicity of b[a]a2G and b[a]a6A with the analogous but simpler benzylated derivatives of guanine and adenine [N2-benzyl-2'-deoxyguanosine (bn2G) and N6-benzyl-2'-deoxyadenosine (bn6A)].
The mutagenicity of b[a]a2G and b[a]a6A in human cells was substantially higher than in E. coli (Fig. 4, 5). Several studies have reported the marked differences in the mutagenic potency of the exocyclic DNA adducts between E. coli and mammalian cells (9~11). 1,N6-ethenodeoxyadenosine was highly mutagenic in mammalian cells but marginally mutagenic in E. coli (9). 1,N2-ethenodeoxyguanosine, on the other hand, was highly mutagenic in E. coli but only weakly mutagenic in mammalian cells. The converse relationship was observed for 3,N4-ethenodeoxycytidine (10). The present study results together with these reports suggest that the mutation frequency is markedly influenced by host cells.
In addition, the bulkier b[a]a2G and b[a]a6A were significantly more mutagenic than simpler adducts (bn2G and bn6A) in human cells. This indicates that the size of the exocyclic amino group substituent has profound effect on the mutagenic potency of bulky adducts in the site-specific mutagenesis. These data broaden our understanding of the mechanism of substituted structure influencing the mutagenicity of the adduct with bulky exocyclic amino-substitution in human cells.
The mutaton induced by 7-BrMeBA was examined in the supF gene of the plasmid pS189 previously (20). Ad293 cells were transfected with the 7-BrMeBA-modified pS189 DNA and the plasmid was extracted. The extracted DNA was used to transform the E. coli MBM 7070 cells. In this evaluation system, the mutagenic potency of 7-BrMeBA was substantial and the predominant base substitution was the G→T transversion. The present study results are in agreement with these reported results (Table 1).
The sequence context effect (21~24) and efficient repair (16~18) have been assumed as the causality of the failure of these bulky aralkylated adducts [bn2G, bn6A, b[a]a2G and b[a]a6A] to induce significant mutation in E. coli. However, the adducts have the strikingly different mutagenic potency in E. coli and human cells. The significant mutagenicity of these adducts may be intrinsic to their structure in human cells.
CONCLUSIONS
In this human cell site-specific mutagenesis system, bn6A was not mutagenic and bn2G was only weakly mutagenic. In contrast, b[a]a2G and b[a]a6A were highly mutagenic. In addition, bulkier b[a]a2G or b[a]a6A exhibit significantly greater mutagenicity in human cells than in E. coli, which further emphasize the importance of studying the site-specific mutagenesis by carcinogen-modified DNA bases in human cells.