Expression of Cyclooxygenase (COX)-2 as a Prognostic Factor in Nasopharyngeal Cancer
Article information
Abstract
Purpose
To evaluate the relationship between treatment failure and COX-2 expression in nasopharyngeal cancer patients treated with chemotherapy and radiotherapy.
Materials and Methods
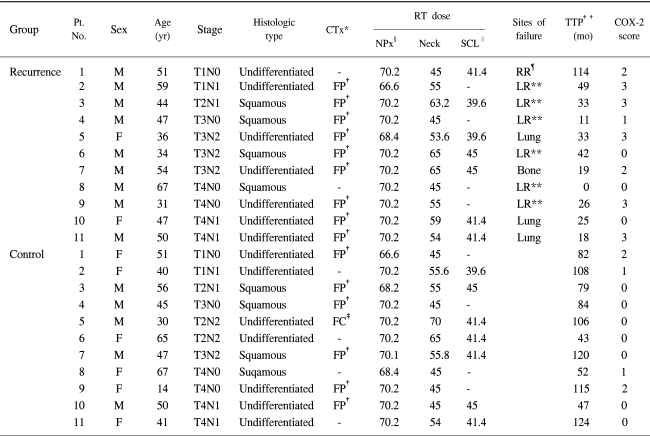
The subjects of this study were 22 nasopharyngeal cancer patients. The patients were treated with neoadjuvant chemotherapy, followed by radiotherapy, or with radiotherapy alone. The formalin-fixed, paraffin-embedded tissues of 11 patients who developed a locoregional recurrence (n=7) or distant metastasis (n=4) were compared with those of 11 disease free patients. Prognostic factors, including histological type, stage, radiation dose and chemotherapy, were well balanced between the two groups. The COX-2 expression was determined immunohistochemically.
Results
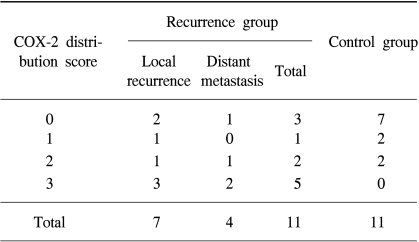
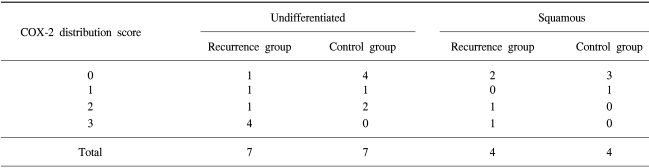
COX-2 expression was stronger in the patients with a locoregional recurrence or distant metastasis than in those free of disease. The COX-2 distribution scores of the control group were as follows: 0 in 7, 1 in 2 and 2 in 2 patients. In the recurrence group, the scores were as follows; 0 in 3, 1 in 1, 2 in 2 and 3 in 5 patients. COX-2 expression was shown to have a statistically significant influence on the treatment failure by the Mann-Whitney U test (p=0.024) and Mantel-Haenszel Chi-Square test (p=0.018). It also significantly influenced the treatment failure when an analysis was performed within patients with a undifferentiated histology (p=0.039 by the Mann-Whitney U test, p=0.037 by the Mantel-Haenszel Chi-Square test).
Conclusion
COX-2 expression is believed to be one of the important factors associated with a locoregional recurrence or distant metastasis.
INTRODUCTION
Head and neck cancer, including the nasopharynx, is one of the diseases where radiotherapy plays an important treatment role. Nasopharyngeal cancer is especially difficult to remove surgically, so radiotherapy has been the main therapeutic option. Recently, several randomized controlled trials have shown survival improvement by combining chemotherapy with radiotherapy (1,2). However, about half the patients experience relapses, despite the combination of radiotherapy and chemotherapy. In general, in the advanced stages the treatment results in a poor outcome, but the tumor behavior can be difficult to predict, with some being biologically more aggressive and more refractory to treatment than others at the same stage and with the same histology. Consequently, the treatment outcome of these patients does not always correlate with the stage, which is the most important prognostic factor. Recently, COX-2 expression appeared to predict a shorter survival in patients with head and neck cancer (3).
Two isoforms of COX have been characterized. COX-1 is the constitutive isoform present in most of normal tissues, and mediates the synthesis of the prostaglandins (PGs) required for normal physiologic functions, such as the cytoprotection of gastric mucosa and the control of platelet aggregation. In contrast, COX-2 is not detected in most tissues, but is induced by oncogenes, growth factors, cytokines and tobacco carcinogens (4). Several studies have revealed that COX-2 expression is up-regulated in human tumors, including head and neck cancers as well as breast, gastric and colorectal cancers (5~8). Moreover, COX-2 expression was considered to be related with a poor prognosis in several types of tumors, such as lung, esophageal, stomach, colorectal and uterine cervix cancers (9~13). In this study, the possibility of a relationship between COX-2 and treatment failure in nasopharyngeal cancer patients was investigated.
MATERIALS AND METHODS
1) Patients and treatment
Among the nasopharyngeal cancer patients who had undergone radiotherapy at Seoul National University Hospital since 1993, 11 who developed a local recurrence (n=7) or distant metastasis (n=4) were enrolled as a study arm (recurrence group). For matching, 11 patients not experiencing a recurrence were randomly selected for comparison (control group). The matching factors were histological type, T and N stage, radiation dose and chemotherapy.
The primary mass was given up to 70.2 Gy, with the exception of two cases in each group. The dose to the lower neck (including supraclavicular lymph node) ranged from 39.6 to 45 Gy, and involved lymph nodes from 54 to 70 Gy, according to extent of the disease and the response to treatment. Most patients (9 of 11 in the recurrence group and 7 of 11 in the control group) were treated with neoadjuvant chemotherapy followed by external beam radiotherapy. The neoadjuvant chemotherapy consisted of 3 cycles of 5-fluorouracil and cisplatin (FP). Only one patient in the control group received 5-fluorouracil and carboplatin (FC). The patient characteristics are summarized in Table 1.
2) Immunohistochemical staining for COX-2
Immunohistochemical staining was performed with 4-µm, formalin-fixed, paraffin-embedded tissue samples. After incubating the slide sections attached on a silane-coating slide, overnight at 37℃, the tissue sections were deparaffinized in xylene (3×10 min) and rehydrated through a series of graded alcohols (100%, 90%, 80%) to diluted water. The deparaffinized sections were then heated and boiled (2×6 min) by microwaving in a 0.01-M citrate buffer (pH 6.0) to retrieve the antigens. To reduce nonspecific staining, each section was treated with 3% H2O2 for 10 min. The sections were then reacted with mouse monoclonal anti-COX-2 (BD Transduction Laboratories, Franklin Lakes, NJ) (1 : 50) for 60 min at room temperature, immersed in TBS Tween 20 (Tris-buffered saline and Tween 20: pH 7.4±0.05, Tris 0.005 M, NaCl 0.15 M, Tween 20 0.05%; 3×5 min) to remove any remaining peroxidase and treated with secondary antibodies (DAKO, Glostrup, Denmark). The peroxidase binding sites were detected by staining with diaminobenzidine (DAB; DAKO), and the sections finally counterstained with Mayer's hematoxylin and observed under a light microscope.
The COX-2 distribution was scored as: 0, no staining; 1, < 10% of cells staining positive for COX-2; 2, 10~50%; and 3, > 50%. The scoring was performed by a single pathologist (Kim CJ) at our institute. Immunohistochemistry was assessed without any information of the patient outcome.
3) Statistics
The patient characteristics for the two groups were compared using Fisher's exact tests. The Mann-Whitney (MW) U and Mantel-Haenszel (MH) Chi-Square tests were used to examine the relationship between the COX-2 expression and prognosis.
RESULTS
The prognostic factors of treatment outcome, such as histological type, T and N stage, radiation dose and chemotherapy, were similar in both groups. The median time of follow-up for patients who remained free of disease was 84 months (range: 43~124 months).
1) COX-2 expression as a prognostic factor
COX-2 staining is cytoplasmic, as shown in Fig. 1 COX-2 expression was higher in the recurrence than in the control group. None of the 11 patients in the control group had a COX-2 distribution score of 3, compared to 5 of the 11 (46%) in the recurrence group (Table 2). This difference in COX-2 distributions between the two groups was statistically significant by both the MW U (p=0.024) and the MH Chi-Square tests (p=0.018).
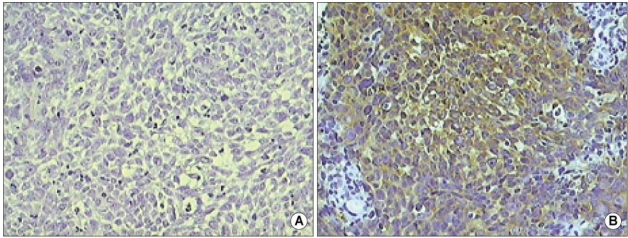
The examples of COX-2 staining in an undifferentiated carcinoma of the nasopharynx. (A) Negative for COX-2 staining (COX-2 score 0)(original magnification ×200). (B) Strongly positive cytoplasmic staining (COX-2 score 3)(original magnification ×200).
According to the type of treatment failure, there were 7 locoregional recurrences and 4 distant metastases. The patients with a locoregional recurrence had a higher COX-2 distribution score compared with those in the control group (p=0.042 by the MH Chi-Square test, p=0.064 by the MW U test). The COX-2 distribution score was higher in patients with distant metastasis than in those free of disease (p=0.032 by the MH Chi-Square test, p=0.064 by the MW U test).
2) COX-2 expression according to the histologic type
According to the histological type, there were 7 undifferentiated carcinomas and 4 squamous cell carcinomas in each group (Table 3). The COX-2 expression also significantly influenced the treatment failure by both the MW U (p=0.039) and the MH Chi-Square tests (p=0.037) when the analysis was performed within the patients with undifferentiated histology. In patients with a squamous cell carcinoma, no patients in the control group had COX-2 distribution scores of 2 or 3, compared with two in the recurrence group. However, the numbers in the study were too small, so no association of COX-2 expression and treatment failure could be concluded in the patients with a squamous histology.
DISCUSSION
COX-2 is well known to have an important role in the carcinogenesis in various types of cancer. Early investigations have shown the relationship of COX-2 inhibitors and its possible use as a chemopreventive agent. It has been reported that aspirin intake might reduce the incidence and mortality of colon and esophageal cancers (14,15). In addition, several reports have demonstrated that selective COX-2 inhibitors suppress carcinogenesis in experimental animals (16). In a human trial as well as an animal model, the chemopreventive effects of selective COX-2 inhibitors were established in a precancerous condition (17). Recently, there have been several direct evidences that support the association of COX-2 with carcinogenesis. The importance of COX-2 activity during carcinogenesis has been demonstrated in both the esophagus and lung (18,19). By knocking out the COX-2 gene, the numbers and size of intestinal polyps were markedly reduced in an animal model of FAP (20).
A relationship between COX-2 and head and neck cancer has also been reported. COX-2 expression was shown to be elevated in head and neck squamous cell carcinomas through quantitative RT-PCR and Western blotting, as well as immunohistochemical staining (5), and was also elevated in dysplasia of the head and neck (21).
COX-2 expression as a prognostic factor has been established in several types of tumor (9~13). In head and neck carcinomas, the prognostic significance of COX-2 has also been demonstrated. Patients with increased expression of COX-2 had a significantly shorter overall survival (3). However, this study included only a squamous histology, and various primary sites of the head and neck.
In the present study, a high level of COX-2 expression was found to correlate with treatment failure in nasopharyngeal cancer. Despite the small sample size (n=22) of this study, the difference was statistically significant.
When the analysis was confined to patients with an undifferentiated histology, the prognostic significance of COX-2 expression was maintained. To the best of our knowledge, this is the first report that demonstrates the relationship of COX-2 expression and poor prognosis in an undifferentiated histology.
These evidences implicated that the use of COX-2 inhibitors might have a potential role in decreasing local recurrence and distant metastasis in nasopharyngeal cancer. Recently, several reports have suggested that COX-2 inhibitors might enhance the effect of radiotherapy (22,23) and inhibit the tumor growth on its own (23,24) in animal models. Based on these evidences, COX-2 inhibitors may be considered as a potential adjuvant treatment in patients with nasopharyngeal cancer.
CONCLUSIONS
COX-2 expression may be one of the prognostic factors in patients with nasopharyngeal cancer.
Notes
This study was supported by Korea Foundation for Cancer Research Grant NO. 23-15-2.