Identification of CLP36 as a Tumor Antigen that Induces an Antibody Response in Pancreatic Cancer
Article information
Abstract
Purpose
Pancreatic cancer has a poor prognosis, due in part to the lack of an effective approach for its early detection. The identification of tumor antigens potentially provides a means for the early diagnosis. The purpose of this study was to use a proteomic approach for the identification of proteins that commonly induce a humoral response in patients with pancreatic cancer.
Materials and Methods
Proteins from the pancreatic adenocarcinoma cell line, BxPC3, were subjected to two-dimensional polyacrylamide gel electrophoresis, followed by Western blot analysis, where individual sera were tested for autoantibodies. Sera from 36 patients with pancreatic adenocarcinoma, and 68 from control groups (14 from lung adenocarcinoma, 19 from colon adenocarcinoma and 35 from healthy subjects) were analyzed. CLP36 expression was evaluated by immunohistochemical analysis and real-time PCR. The cellular localization of CLP36 as an autoantigen was investigated by Western blot analysis.
Results
The autoantibody was detected against a protein, identified by mass spectrometry as CLP36, in 14 of the 36 sera (38.9%) from patients with a pancreatic adenocarcinoma, and 3 of the 68 controls (4.4%). Immunohistochemical analysis of CLP36 in a tissue array demonstrated diffuse and consistent immunoreactivity in the pancreatic adenocarcinomas. The levels of CLP36 mRNA were highest in the pancreatic cancer cell lines of the different cells analyzed. The molecular weight of the protein displayed in the membrane-rich fraction was larger than that in the cytosolic fraction, which is likely attributable to a post-translational modification.
Conclusion
CLP36 was identified as a tumor autoantigen inducing a humoral immune response in pancreatic adenocarcinomas. More detailed studies need to be undertaken to understand whether the humoral response by CLP36 is tumor-specific.
INTRODUCTION
There is a substantial evidence for a humoral immune response to cancer in humans, as shown by the identification of autoantibodies to a number of intracellular and surface antigens in patients with different tumor types (1~3). Harnessing the immune response to identify novel cancer biomarkers is an attractive strategy, because the immune system performs biological amplification with antigenic tumor proteins as templates, beginning at an early stage during tumor development when the tumor may be otherwise undetectable (4). A tumor-specific humoral immune response directed against overexpressed oncoproteins (5,6), mutated proteins, such as p53 (7), or other aberrantly expressed proteins, has been previously described. It is currently largely unknown whether the occurrence of such antibodies is beneficial. However, knowledge of potential tumor antigens potentially evoking immune responses during tumorigenesis may have relevance to the development of an effective cancer immunotherapy.
Autoimmunity in pancreatic cancer has been demonstrated against several proteins, including MUC1 (8), p53 (8) and Rad51 (9) proteins. These autoantibodies, however, have been observed in only 7~20% of patients with pancreatic cancer. Furthermore, many of the tumor autoantigens were identified by an immunoassay, such as ELIZA, with sera from cancer patients to determine the immunogenicity of the previously known autoantigens in various autoimmune diseases. Therefore, the screening of tumor autoantigens from the whole cell proteome would be necessary to enhance and validate a specific serological marker and its corresponding native antigen.
We have implemented a proteomic approach for the identification of tumor antigens that elicit a humoral immune response in pancreatic adenocarcinoma. In this study, the pancreatic cancer cell line, BxPC3, was utilized as the source of tumor proteins for antigen identification, with two-dimensional gel electrophoresis (2-D PAGE) to separate protein constituents, followed by their transfer onto membranes. Sera from cancer patients and control groups were screened individually for antibodies that react against separated proteins by Western blot analysis. Mass spectrometric analysis was used for protein identification. In this study, the identification of CLP36 as an autoantigen eliciting a humoral immune response in pancreatic cancer is reported.
MATERIALS AND METHODS
1) Sera and cell lines
Serum and tumor tissue were obtained at the time of diagnosis after obtaining the informed consent of the subjects. The experimental protocol was approved by The University of Michigan Institutional Review Board. Sera were obtained from 36 patients with a pancreatic adenocarcinoma, and from 35 healthy individuals and 33 patients with other cancers (14 with lung cancer and 19 with colon cancer) which were used as controls. The human pancreatic cancer cell line, BxPC3, was cultured in RPMI 1,640 medium supplemented with 10% fetal bovine serum, and 100 units/ml each of penicillin and streptomycin (Invitrogen).
2) 2-Dimensional polyacrylamide gel electrophoresis
Cultured BxPC3 pancreatic carcinoma cells were harvested in 300 µl of solubilization buffer, using a cell scraper, and stored at -80℃ until used. Proteins derived from the extracts of cultured cells were separated by 2-D PAGE, as described previously (10). Briefly, solubilized proteins were applied to isoelectric focusing gels, with focusing performed using pI 4 to 8 carrier ampholytes at 700 V for 16 h, followed by 1,000V for an additional 2 h. Following equilibration in loading buffer (125 mM Tris, pH 6.8, 10% glycerol, 2% SDS, 1% dithiothreitol and bromophenol blue), the first-dimension gel was loaded into a cassette containing the second-dimension gel. For the second-dimension separation, a gradient of 11 to 14% acrylamide (Crescent Chemical, Hauppauge, NY) was used. Proteins were transferred to a PVDF membrane (Millipore, Bedford, MA) or directly visualized by staining of the gels.
3) Western blotting
Following transfer, the membranes were incubated with blocking buffer, consisting of Tris-buffered saline, 1.8% nonfat dry milk and 0.05% Tween 20, for 2 h. The membranes were individually incubated for 1.5 h at room temperature, with serum obtained from either a patient with pancreatic cancer or a control at a 1:100 dilution. After three washes with washing buffer (Tris-buffered saline containing 0.05% Tween 20), the membranes were incubated with horseradish peroxidase-conjugated anti-human IgG antibodies (Amersham), at a dilution of 1:1,000, for 30 min at room temperature. Immunodetection was accomplished by ECL (Enhanced Chemiluminescence (Amersham)) followed by autoradiography on Hyperfilm MP (Amersham).
4) CLP36 detection by Western blotting
A rabbit anti-CLP36 polyclonal antibody was kindly provided by Dr T. Makela (Haartman Institute and Helsinki University Central Hospital, Biomedicum Helsinki, University of Helsinki, Finland). The antibody was used at a 1:2,000 dilution for Western blotting, and processed as for the incubations with patient sera, using horseradish peroxidase-conjugated anti rabbit IgG (Amersham) as secondary antibody.
5) In-gel enzyme digestion and mass spectrometry
For protein identification by mass spectrometry, 2-D gels were stained by a modified silver staining method, and excised proteins destained for 5 min in 15 mM potassium ferricyanide and 50 mM sodium thiosulfate, as previously described (11). Following three washes with water, the gel pieces were dehydrated in 100% acetonitrile for 5 min and then dried. Digestion was performed by the addition of 100 ng of trypsin (Promega, Madison, WI) in 200 nM ammonium bicarbonate. Following enzymatic digestion, overnight at 37℃, the peptides were extracted twice with 50 µl of 60% acetonitrile/1% trifluoroacetic acid. Following removal of the acetonitrile by centrifugation in a vacuum centrifuge, the peptides were concentrated using C18 pipette tips (Millipore, Bedford, MA) and identified by nanoflow capillary liquid chromatography coupled with electrospray quadrupole-time of flight tandem mass spectrometry (ESI Q-TOF MS/MS) in the Q-TOF micro (MicroMass, Manchester, UK). The acquired spectra were processed and searched against a non-redundant Swiss-Prot protein sequence database using the proteinLynx Global Server (www.micromass.co.uk).
6) RNA isolation
Human tumor cell lines were homogenized in the presence of TRIzol reagent (Life Technologies Inc., Gaithersburg, MD) and the total cellular RNA purified according to manufacturer's procedures. RNA samples were further purified using acid phenol extraction and RNeasy spin columns (Qiagen, Valencia, CA), and the quality assessed by 1% agarose gel electrophoresis in the presence of ethidium bromide.
7) Determination of CLP36 mRNA levels using real-time PCR
Five pancreatic, 4 lung, 4 colon and 2 ovarian cancer cell lines were used to compare the levels of CLP36 mRNA expression after normalizing with GAPDH mRNA. Oligonucleotide primers and TaqMan probes were designed using the Light Cycler Probe Design Software (Roche Applied Science). The forward and reverse primers for human CLP36 were 5'-AGCGTCATCCATACAAG-3' and 5'-TGGTCTAAGGGTCTGC-3', respectively, and those for GAPDH were 5'-GAAGGTGAAGGTCGGAGTC-3' and 5'-GAAGATGGTGATGGGATTTC-3', respectively. All oligonucleotide primers were purchased from Applied Biosystems.
The first-strand cDNA was synthesized using the SuperScript First-Strand Synthesis System for RT-PCR, according to the manufacturer's instructions (Invitrogen). Quantitative PCR reactions were carried out in 96-well optical reaction plates, using cDNA equivalent of 50 ng of total RNA for each sample, in a volume of 25 µl. PCR was performed on the ABI Prism 7700 Sequence Detector (Applied Biosystems). The cycling conditions were 10 min at 95℃, followed by 55 cycles at 95℃ for 30 sec, 60℃ for 45 sec and 72℃ for 45 sec.
To control the variation in the amount of starting RNA in the samples, amplification of the GAPDH mRNA was performed, as an internal reference, against which the other RNA values were normalized. The real-time PCR products were purified using a QIAQuick Gel Extraction Kit (Qiagen) and subjected to DNA sequencing to verify the identity of the real-time PCR products.
8) Pancreas/ampullary tissue array and immunohistochemistry
A tissue array containing triplicates of 4 normal pancreas, 12 non-pancreas normal tissues, 78 pancreatic and ampullary adenocarcinomas and 2 large anaplastic carcinomas was constructed, as previously described (12). Immunohistochemistry for CLP36 was performed using the same rabbit polyclonal antibody (30-minute incubation at RT), at a dilution of 1:100 using Tris buffer (pH 9.0), with microwave antigen retrieval (15 minutes). The primary antibody was detected using the Dako Envision kit.
9) Western blotting of cytosolic and membrane-rich fractions with CLP36 antibody
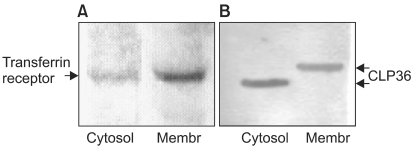
Both the cytosolic and membrane-rich fractions were analyzed to determine the subcellular location of the CLP36 as an autoantigen. To obtain the cytosolic and membrane-rich fractions of BxPC3 whole cell lysates, the cells were lysed in a buffer containing 50 mM Tris-HCl (pH 7.4), 0.32 M sucrose, 1mM EDTA, protease inhibitors and 10 mM iodoacetamide. The cells were passed 15 times through a 25-gauge needle and centrifuged at 20,000×g for 2 min at 4℃, after which the supernatant was collected. The supernatant was again centrifuged at 100,000×g for 20 min at 4℃. The resulting supernatant (cytosolic fraction) and pellet (membrane-fraction) were collected. The membrane fraction was solubilized in homogenization buffer containing 1% Triton-X 100. The fractions were resolved by 4~15% SDS-PAGE and transferred onto a nitrocellulose membrane. A transferrin receptor (CD 71) antibody (Santa Cruz Biotechnology) was used as a positive control for the membrane-rich fraction.
RESULTS
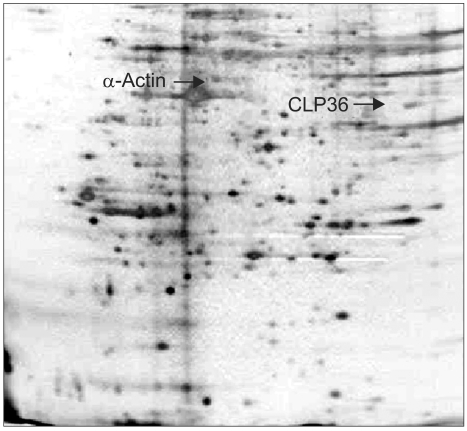
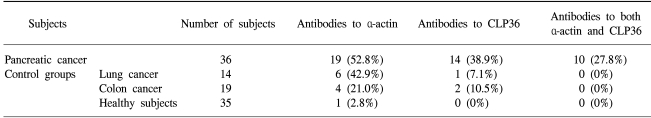
1) Autoantibodies to a pancreatic tumor protein in sera from patients with pancreatic cancer
The sera obtained from the 36 newly diagnosed patients with pancreatic adenocarcinoma, 33 patients with other types of cancers and the 35 healthy donors were screened individually for the presence of antibodies to BxPC3 proteins (Fig. 1). α-Actin, a well recognizable landmark in 2-D patterns, exhibited frequent reactivity with the sera of cancer patients relative to those of the controls, but exhibited no specificity for pancreatic cancer (Table 1). Another polypeptide, with an estimated molecular mass of 36 kDa and pI of 7.5, showed reactivity with sera from 14/36 (38.9%) patients with pancreatic adenocarcinomas and 3/33 (9.1%) of the other cancer patients, but no reactivity with the sera from the 35 healthy donors (Table 1). No further protein spots on the 2-D gels showed specific reactivity against the sera from the cancer patients compared with those from the healthy subjects, so these two spots were evaluated.
2) Identification of the immunoreactive proteins as α-actin and CLP36
Both the presumed α-actin and second reactive spots were excised from the silver-stained 2-D PAGE gels. The proteins were digested with trypsin, and the resulting peptides analyzed by ESI Q-TOF Tandem MS spectrometry. The acquired spectra were processed and searched against a non-redundant Swiss-Prot protein sequence database using the proteinLynx Global Server (www.micromass.co.uk). The identity of α-actin was confirmed (data not shown), and the protein in the second reactive spot identified as CLP36 (Fig. 2). The identity of this protein was further confirmed by 2-D Western blotting using BxPC3 whole-cell extracts and the anti-CLP36 rabbit polyclonal antibody (Fig. 3).
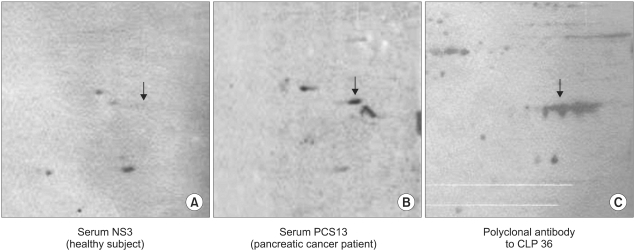
Western blot analysis of CLP-36 from the sera of a healthy individual (A), of a pancreatic cancer patient (B) and with a polyclonal anti-CLP36 antibody (C).

Tandem mass spectrometry identification of CLP36 after trypsin digestion. The MS/MS spectrum of CLP36 obtained after trypsin digestion is shown by analysis with ESI-Q-TOF, coupled with nanoflow capillary high-performance liquid chromatography. The precursor ion shown in the figure has an m/z of 1551.1383, and the peaks were searched against the non-redundant SwissProt protein sequence database using the ProteinLynx global server. A total of six tryptic peptides, as shown, matched the CLP36 protein.
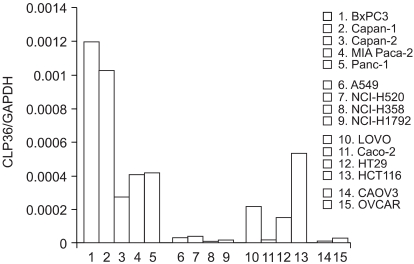
3) RNA levels of CLP36 in cancer cell lines
To try and determine the RNA level for the CLP36 gene, real-time PCR was performed. For normalization with the expression of the endogenous gene, the CLP36/GAPDH ratio was calculated from different cancer cell lines (Fig. 4). The ratio of CLP36/GAPDH measured in the BxPC3 was 1.2×10-4, the highest expression level among the different cell lines tested. Other pancreatic cancer cell lines also exhibited a relatively high ratio. Three of the 4 colon cancer cell lines showed high CLP36 mRNA levels. The CLP36 mRNA levels in the four lung and two ovarian cancer cell lines tested were relatively reduced in comparison to the other cell lines.
4) Immunohistochemical evaluation of CLP36 expression
The CLP36 protein expression was investigated using immunohistochemistry and a pancreas/duodenal adenocarcinoma tissue array. Diffuse and consistent cytoplasmic immunoreactivities were observed in the large majority of pancreatic and ampullary adenocarcinomas (Fig. 5). Immunoreactivities were also observed in normal endothelial cells and various other normal tissues (not shown).
5) Localization of CLP36 as a tumor autoantigen
The cytosolic and membrane-rich fractions were analyzed to determine the distribution of CLP36 showing immunoreactivity with the sera from pancreatic cancer patients. The transferrin receptor antibody, which was utilized as a positive control for plasma membrane protein, reacted strongly with the membrane-rich fraction. The rabbit anti-CLP36 antibody showed reactivity with both the cytosolic and membrane-rich fractions, but the molecular weight of the protein displayed in the membrane-rich fraction was higher than that in the cytosolic fraction (Fig. 6), which is likely attributable to a post-translational modification. To determine whether CLP36 was post-translationally modified by glycosylation, a putative cause of autoantigenicity, the whole cell lysate of BxPC3 was simultaneously treated with N-glycosidase F, Endo-α-N-acetylgalactosaminidase and α-2-3,6,8,9-Neuraminidase. BxPC3 whole cell lysates were separated by 1-D SDS PAGE and transferred to PVDF membrane for Western blotting with anti-CLP36 antibody. As the deglycosylating enzyme treatment resulted in no mobility shifts of the CLP36 in the SDS-polyacrylamide gels (data not shown), it was concluded that the CLP36 present in the membrane fraction was not glycosylated.
DISCUSSION
The identification of circulating tumor antigens eliciting a humoral response, or their related autoantibodies, provides a potential means for early cancer diagnosis. The proteomic approach for the identification of tumor antigens in pancreatic cancer uncovered antibodies against a 36-kDa protein, identified as PDZ, and a LIM-domain protein, CLP36, in 14 of the 36 sera. The CLP36 antigen was not detected in any of the non-cancer control sera tested, and only 3 of the 33 sera from the different tumor patients exhibited IgG immunoreactivity to CLP36.
The LIM domain, a double zinc finger structure, is thought to be involved in protein-protein interactions rather than DNA-protein interactions (13). Because, of the protein interacting capabilities, LIM domains are; therefore; thought to be important for the targeting of proteins to specific subcellular locations and in mediating the assembly of multi-protein complexes (14). Carboxyl terminal LIM domain proteins have been shown to be primarily cytoplasmic and have functions related to the cytoskeleton and signal transduction pathway (15,16).
Raedle et al. (8) analyzed the sera of pancreatic cancer patients to determine the occurrence of autoantibodies to the p53 protein. Anti-p53 autoantibodies were detected in 6 out of 33 cases, but they were not specific for malignancy, suggesting that severe inflammatory processes may also induce anti-p53antibodies. Even these autoantibodies were not specific for malignancy, but may indicate a risk for the development of pancreatic cancer.
It is of interest that previously, CLP36 was found localized to actin stress fibers via its PDZ domain through its association with cellular α-actinin-1 and α-actinin-4, suggesting that CLP36 acts as an adapter between stress fibers and LIM-binding proteins in non-muscle cells (17). α-Actinin-4 is an actin-bundling protein associated with cell motility, endocytosis and cancer invasion (18). Recently, a point mutation in the α-actinin-4 gene was found to generate an antigenic peptide recognized by autologous cytolytic T lymphocytes (CTL) in human lung carcinomas (19). Actin is one of the basic components forming the cell cytoskeleton, an observation consistent with the widespread reaction of smooth muscle antibody against numerous cell types seen in patients with chronic active hepatitis. Anti-actin autoantibodies have been detected in chronic liver disease (20,21) and gastric cancer (22). Of interest, the sera of 19 of the 36 pancreatic cancer patients in our study showed reactivity against α-actin, and 10 of the 14 sera that showed reactivity against CLP36 also reacted with α-actin, suggesting the autoantigenicity of CLP36 is likely to be evoked by α-actin.
Most antigens recognized by autoantibodies are molecules existing within cells under normal conditions. However, the structure and/or subcellular localization of a number of molecules change during or after cell death. These molecules are recognized by the immune system, leading to the development of autoimmunity when the dead cells fail to be effectively cleared by phagocytosis (23). In fact, some autoantigens, such as the nuclear autoantigen La or ribonucleoprotein Ro, move to a region near the cell periphery during apoptosis (24). Thus, it can be proposed that the translocation of cellular antigens to the plasma membrane could have a causative role in autoimmunity. In the present study, the cytosolic and membrane-rich fractions were analyzed to determine the subcellular location of CLP36. CLP36 was localized in both the membrane and cytosol fractions. Even the transferrin receptor, a positive control for plasma membrane proteins, was strongly detected in the membrane-rich fraction; it would be worth investigating the purification of the surface membrane fraction for the CLP36 autoantigen localization.
The CLP36 from the membrane-rich fraction had a different molecular weight, which is likely attributable to a post-translational modification. Recently, a number of biochemical modifications arising in proteins after their translation have been shown to be a common event in autoantigen proteins (25). No evidence for CLP36 glycosylation was found in this study, but other types of modification have not been ruled out.
CONCLUSIONS
Our data have demonstrated that the anti-CLP36 antibody was detected in the sera from pancreatic cancer patients, but not in any of the non-cancer control sera. Therefore, whether anti-CLP36 antibodies occur during tumor development or progression, or occur at an earlier pre-malignant stage; for example, as part of a severe inflammatory process, remain to be determined. The mechanism for CLP36 autoantigenicity also needs to be uncovered. It is clear; however, that the proteomic approach allowing for the screening of native proteins as they are expressed in tumor cells has the potential to identify novel proteins with potential clinical utility in cancer.