ERRATUM: Recommendations for the Use of Next-Generation Sequencing and the Molecular Tumor Board for Patients with Advanced Cancer: A Report from KSMO and KCSG Precision Medicine Networking Group
Article information
Correction to: Cancer Res Treat. 2022 Jan;54(1):1-9; DOI: https://doi.org/10.4143/crt.2021.1115
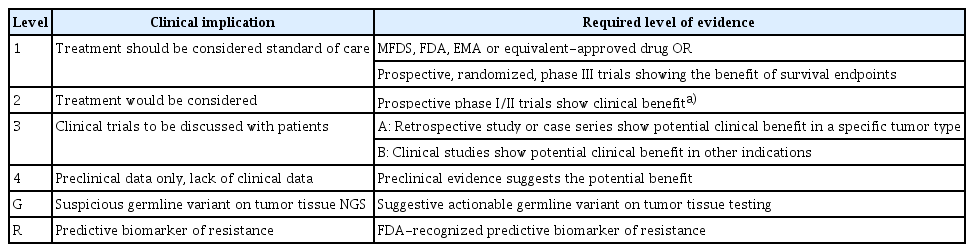
For the data represented in Table 2, we have corrected the level of evidence of K-CAT level 2 and 3. As the table shown, prospective phase I/II trials required for K-CAT level 2 include clinical trials across tumor types, such as basket trials. For the clinical benefit of specific cancer types, expert consensus is needed. K-CAT level 3A requires a retrospective study or case series with potential clinical benefit in s specific tumor types. K-CAT level 3B is revised from a retrospective study as clinical studies show potential clinical benefits in other indications. The corrected version of the table is below.