Pattern of Apoptosis by NS398, a Selective COX-2 Inhibitor, in Hepatocellular Carcinoma Cell Lines
Article information
Abstract
Purpose
NS398, a selective COX-2 inhibitor, is known to inhibit the growth of COX-2 expressing hepatocellular carcinoma cells. The present study investigated whether the cytotoxic effect of NS398 was COX-2 dependent and whether caspases were involved in NS398-induced apoptosis in hepatocellular carcinoma cells.
Materials and Methods
The expressions of COX-2 in SNU 423 and SNU 449 hepatocellular carcinoma cell lines were examined using RT-PCR and Western blot. The cytotoxic effect of NS398 was measured using MTT in the presence or absence of caspase inhibitors. The distribution of the cell cycle and extent of apoptosis were analyzed using flow cytometry and a Cell Death Elisa kit, respectively.
Results
The expression of COX-2 was observed in SNU423 cells, but not in SNU 449 cells. NS398 treatment resulted in both dose-and time-dependent growth inhibitions, with increases in apoptotic cells in both cell lines. Treatment with the pan-caspase inhibitor, z-VAD- fmk, or the caspase-3 inhibitor, Ac-DMQD-CHO, showed no attenuation of the cytotoxic effect of NS398 in either cell line.
Conclusion
This study demonstrated that the cytotoxic effect of NS398 was independent of COX-2 expression. Caspases were also shown not to be involved in NS398-induced apoptosis in either SNU 423 or SNU 449 Korean HCC cell lines. Our data suggests the feasibility of preventing hepatocellular carcinoma with the use of COX-2 inhibitors needs to be carefully evaluated.
INTRODUCTION
Cyclooxygenase (COX) is a key enzyme for the conversion of arachidonic acid to prostaglandins and other eicosanoids. Whereas COX-1 is constitutively expressed, Cyclooxygenase 2 (COX-2) is a highly inducible gene (1). Increased COX-2 expression has been associated with inhibition of apoptosis (2), increased angiogenesis (3) and enhanced metastatic ability (4). Moreover, the overexpression of COX-2 is associated with tumorigenesis in a number of human cancers, including hepatocellular carcinomas (5~7). Very recently, the frequency and level of COX-2 expression in poorly-differentiated hepatocellular carcinoma (HCC) was reported to be similar to that of well-differentiated HCC, suggesting that COX-2 may play a role in the advanced as well as the early stages of hepatocarcinogenesis (7).
The expression of COX-2 in some HCC cell lines, and the growth inhibition in these cell lines by NS398, a selective COX-2 inhibitor, has recently been reported (6~10). In addition, NS398, a selective COX-2 inhibitor, has been demonstrated to inhibit the growth of COX-2 expressing Hep3B human HCC cells via caspase-independent apoptosis (11), suggesting the feasibility of chemotherapy for HCC using COX-2 inhibitors. However, it remains unclear whether the cytotoxic effect of NS398 and caspase-independent apoptosis are COX-2 independent and a general feature of NS398-induced apoptosis, respectively, in human HCC cells. Therefore, in this study, the cytotoxic effects of NS398 in SNU 423 and SNU 449 Korean human HCC cells, expressing and not expressing COX-2, respectively, were examined. In addition, the involvement of caspases in NS398-induced apoptosis were examined in both cell lines.
MATERIALS AND METHODS
1) Cell lines
The SNU 423 and SNU 449 cell lines, derived from human hepatocellular carcinomas (HCCs), were purchased from the Korea Cell Line Bank (Seoul). The Cell lines were cultured in RPMI 1640, with 10% fetal bovine serum (FBS), at 37℃ under 5% CO2.
2) RNA preparation and RT-PCR
Total RNA was isolated from the cells using TRIzol reagent. cDNA was synthesized from 2 µg of total RNA using a cDNA synthesis kit, containing the superscript II reverse transcriptase and random hexamers. Small aliquots of the cDNA product were amplified with gene-specific primer pairs. The primer set for COX-2 was COX-2F (5'-TTGTTGAATCATTCACCAGGC-3') and COX-2R (5'-ACACTGAATGAAGTAAAGGGA-3'). β-actin was amplified as an internal control. The primers for β-actin were β-actin-F (5'-ACCATGGATGATGATATCGC-3') and β-actin-R (5'-ACATGGCTGGGGTGTTGAAG-3'). PCR amplification was performed after initial denaturation of 5 min at 94℃, followed by 30 cycles of 30s at 94℃, 45s at 58℃ and 1 min at 72℃, with final elongation at 72℃ for 10 min.
3) Cell viability assay
Cells were plated overnight, at a density of 5,000 cells/well, in 96-well plates with 10% FBS-supplemented medium. The medium was then changed to serum-free medium, with various concentrations of NS398, and the cells incubated for the indicated times. The 3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay was employed to check the Plymouth Meeting, PA), Z-Val-Ala-Asp-fluoromethylketone (Z-cell viability, as previously described (12). NS398 (Biomol, VAD-fmk; Calbiochem, San Diego, CA), and Ac-Asp-Met-Gln-Asp-CHO (Ac-DMQD-CHO; Calbiochem) were dissolved in DMSO. The final DMSO concentration in the media was 0.1%.
4) Analysis of cell cycle distribution
Cells were exposed to either DMSO or 100 µM NS398 for 72 h. The cells were then collected, washed with PBS and fixed overnight in 70% ethanol at 4℃. The distribution of the cell cycle was analyzed using flow cytometry, as previously described (13).
5) Apoptosis assay
This assay was performed using a Cell Death Elisa kit (Roche, Mannheim, Germany), following the manufacturer's instructions. Briefly, after treatment with 100 µM NS398 for 72 h, cytoplasmic extracts were prepared from 10,000 cells, with the DNA-histone complexes measured by reading the optical density at 405 nm, using a microplate reader.
6) Immunoblot
For the detection of the expression of COX-2 in the SNU 423 and SNU 449 cells, total cell lysates were prepared and the protein concentrations measured using the BCA protein assay (Pierce, Rockford, IL). Protein samples (30 µg/lane) were separated on 10% polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were blocked with 6% milk in PBS for 1 h, and then probed with polyclonal anti-COX-2 antibodies (Amersham, Buckinghamshire, UK) at a 1:5,000 dilution. Horseradish peroxidase (HRP)-conjugated donkey anti-rabbit Ig (Amersham) was used as the secondary antibody at a 1:5,000 dilution. Bands were visualized using ECL Plus (Amersham). Probing with the monoclonal anti-actin antibody (Chemicon, Temecula, CA) was used to confirm equal loading and transfer.
RESULTS
To investigate whether the cytotoxic effect of NS398 was COX-2 dependent, SNU 423 and SNU 449 Korean HCC cell lines were selected based on our previous study. The SNU 423 cells expressed relatively strong cytoplasmic COX-2 immunofluorescence staining, whereas the SNU 449 cells showed no detectable staining (7). Moreover, the expression of COX-2 mRNA and protein was observed in SNU 423 cells, whereas the expression in SNU 449 cells was scarcely detected by RT-PCR and Western blot analysis (Fig. 1).
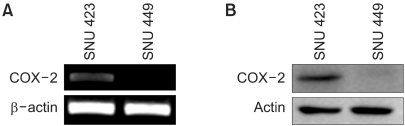
COX-2 expression in SNU 423 and SNU 449 hepatocellular carcinoma cell lines. (A) RT-PCR and (B) Western blot analysis.
NS398 treatment resulted in dose- and time-dependent growth inhibition in both cell lines (Fig. 2). Morphological changes were also observed in both cell lines cultured with NS398: the cells were rounded up and detached from the plate (data not shown). These results clearly show the cytotoxic effect of NS398 to be COX-2 independent. In medium containing 10% serum, the growth inhibition in both cell lines was considerably reduced (data not shown). Analysis of the cell cycle distribution by flow cytometry showed the population of cells in the sub-G1 phase, representing apoptotic cells, increased in response to NS398 treatment in both cell lines (Fig. 3). The extent of apoptosis was also quantified using a Cell Death Elisa Assay, which measures the DNA fragments associated with cytoplasmic histone. Treatment for 72 h with 100 (M NS398 produced 5.1- and 4.7-fold increases in apoptosis in SNU 423 and SNU 449 cells, respectively (Fig. 4). Thus, both flow cytometry and the Cell Death Elisa Assay showed the growth inhibition by NS398 was caused by apoptosis in both cell lines, regardless of COX-2 expression. In medium containing 10% serum, the proportion of apoptotic cells in both cell lines was considerably reduced (data not shown): Whether caspases are involved in NS398-induced apoptosis was then examined in both cell lines. Treatment of both cell lines with the pancaspase inhibitor, z-VAD-fmk, or the caspase-3 inhibitor, Ac-DMQD-CHO, showed no attenuation of the NS398 cytotoxic effect (Fig. 5). Moreover, the caspase-3 activity was not elevated after treatment with NS398 in either cell line (data not shown). In addition, there were no changes in levels of pro-caspase-3 or active caspase-3, as shown by the Western blot analysis. At the same time, no cleaved fragments of PARP were detected (data not shown). Together, these results show that NS398-induced apoptosis is caspase-independent in both SNU 423 and SNU 449 cells.
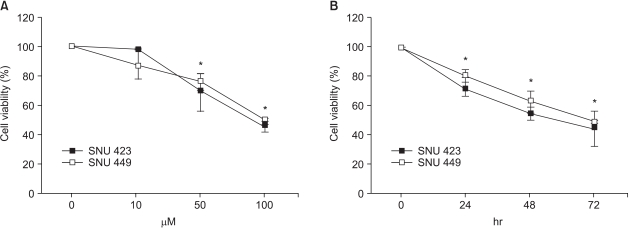
Effect of NS398 on the growth of the SNU 423 and SNU 449 cell lines. (A) Dose-dependent growth inhibition. Cells were treated with NS398 for 72 h. (B) Time-dependent growth inhibition with 100 µM NS398. The MTT assay was employed to measure the cell viability in both cases. Data are the means±standard errors of six determinations per experiment from three independent experiments (*p<0.05).

Cell cycle analysis using flow cytometry. A representative DNA histogram is shown. Cells were exposed to either DMSO or 100 µM NS398 for 72 h.
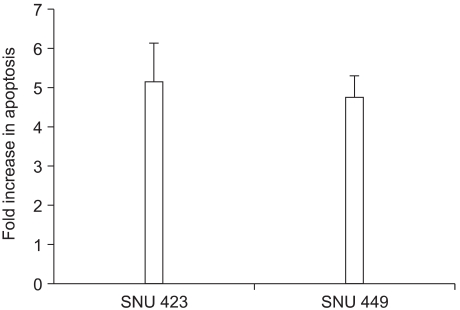
Cell death Elisa assay. Cells were treated with 100 µM NS398 for 72 h. The results are presented as the fold increase in apoptosis relative to the controls that received DMSO only. Data are the means±standard errors of two determinations per experiment from 2 independent experiments.
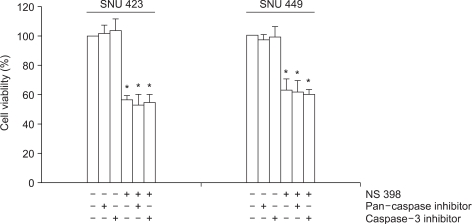
Demonstration of caspase-independence. Cells were treated with 100 µM NS398 for 48 h, in the presence (40 µM) or absence of the pan-caspase inhibitor, z-VAD-fmk, or the caspase-3 inhibitor, Ac-DMQD-CHO. The MTT assay was employed to check the cell viability. Data are the means±standard errors of six determinations per experiment from 3 independent experiments. (*p<0.05).
DISCUSSION
In this study, NS398 was observed to induce apoptosis, not only in the SNU 423 cell line expressing COX-2, but also in the SNU 449 cell line not expressing COX-2. Our data clearly demonstrated the cytotoxic effect of NS398 to be COX-2 independent. Our finding conflicts with the recent report of Cheng et al. (10), who claimed that NS398 does not induce apoptosis in Hep G2 cells, as they detected no expression of COX-2, implying the cytotoxic effect of NS398 to be COX-2 dependent. However, considering Hep G2 is in fact known to express COX-2 (6~9), their conclusions are difficult to accept with respect to the results found in our study.
NS398-induced apoptosis was also observed to be caspase-independent in both SNU 423 and SNU 449 cells. These results suggest that caspase-independent apoptosis may be a general feature of NS398-induced apoptosis in hepatocellular carcinoma cells. Prostate apoptosis response 4 (Par-4), a proapoptotic gene, was up-regulated in HCA-7 human colon carcinoma cells treated with NS398, and has also been suggested as a downstream mediator leading to the initiation of apoptosis (14). However, in this study, no up-regulation of Par-4 was observed in NS398-induced apoptosis in either SNU 423 or SNU 449 cells (unpublished data). Instead, apoptosis- inducing factor (AIF), a novel caspase-independent death effector released from mitochondria (15), was observed to be accumulated in the nuclei of cells treated with NS398 (unpublished data). Further investigation will be needed to elucidate the intracellular signaling pathway leading to apoptosis by NS398.
CONCLUSIONS
This study demonstrates the cytotoxic effect of NS398 to be independent of COX-2 expression. Also, caspases were not involved in the NS398-induced apoptosis in either of the two Korean hepatocellular carcinoma cell lines. Our data suggests that the feasibility of chemotherapy or chemoprevention of HCC by COX-2 inhibitors needs to be carefully evaluated.
Notes
This work was supported in part by a grant (FG00-0101-001-2-2-0) from the 21C Frontier Human Genome Project, the Ministry of Science and Technology, Korea.