Clinical Experience of Patients with Ductal Carcinoma In Situ of the Breast Treated with Breast-Conserving Surgery plus Radiotherapy: A Preliminary Report
Article information
Abstract
Purpose
Breast-conserving therapy (BCT) is a practical alternative to mastectomy for treating ductal carcinoma in situ (DCIS). We reviewed our experience for treating patients with DCIS of the breast to evaluate the outcome after performing breast-conserving surgery plus radiotherapy (BCS-RT).
Materials and Methods
Between January 1983 and December 2002, 25 patients with clinically or mammographically detected DCIS were treated by BCS-RT. One patient was diagnosed with bilateral DCIS. Thirteen cases (50%) had symptomatic lesions at presentation. All 26 cases of 25 patients underwent BCS such as lumpectomy, partial mastectomy or quadrantectomy. All of them received whole breast irradiation to a median dose of 50.4 Gy. Twenty-four cases (92.3%) received a boost to the tumor bed for a median total dose of 59.4 Gy. The median follow up period was 67 months (range: 38 to 149 months).
Results
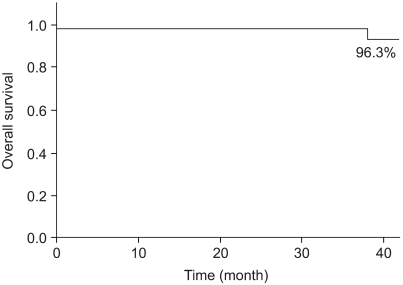
Two cases (7.7%) experienced ipsilateral breast tumor recurrence (IBTR) after BCS-RT. The histology results at the time of IBTR showed invasive ductal carcinoma (IDC), and the median time to IBTR was 25.5 months. On the univariate analysis, there were no significant factors associated with IBTR in the DCIS patients. The three-year local recurrence free survival rate was 96.0% and the overall survival rate was 96.3%.
Conclusion
After the treatment for DCIS, the IBTR rate in our study was similar to other previous studies. Considering that we included patients who had many symptomatic lesions, close or positive margins and less that complete early data, our result is comparable to the previous studies. We could not find the prognostic significant factors associated with IBTR after BCS-RT. A longer follow up period with more patients would be required to evaluate the role of any predictive factors and to confirm these short-term results.
INTRODUCTION
With the widespread use of screening mammography, ductal carcinoma in situ (DCIS) of the breast is being diagnosed with increasing frequency. DCIS is common in western countries and it accounts for more than 21% of the newly diagnosed breast cancer cases (1~3). Most of these patients are asymptomatic and they are primarily detected by screening mammography (3~6). In Korea, DCIS represented 2% to 3% of all breast cancer that was detected from 1980 to 2000 (7,8). The incidence of DCIS has recently increased, but it still shows a lower frequency than that in the western countries (9).
In the early 1980s, most patients with DCIS were treated with mastectomy, and the local recurrence rates were exceptionally low at approximately 1% to 2% (10). Two large prospective randomized trials showed a low rate of local recurrence for the patients who underwent breast-conserving therapy (BCT) for DCIS (11~13). BCT has become an accepted option for the management of most patients with DCIS of the breast. In an effort to optimize the treatment outcome, many researchers have examined the association of various factors with the development of local recurrence (4~6,9~14).
We reviewed our results with performing breast-conserving surgery plus radiotherapy (BCS-RT) for treating patients with DCIS of the breast, and we examined the risk factors for local recurrence and BCS-RT's impact on the outcome
MATERIALS AND METHODS
Between January 1983 and December 2002, we retrospectively reviewed the data of 26 patients who were treated for DCIS of the breast. Until 1990, the patients diagnosed with DCIS of the breast were treated with mastectomy and they were not enrolled in this study. Since 1990, we treated twenty-six patients diagnosed with DCIS with BCT. Twenty-five patients had unilateral DCIS and one patient had bilateral DCIS, for a total of 27 breasts with pathologically confirmed DCIS. One of these patients had previously suffered with contralateral invasive breast cancer. Eight patients had received hormonal replacement therapy for several years. All the cases were treated with BCS followed by radiotherapy. Until 1998, partial mastectomy or quadrantectomy was usually performed. Thereafter, most BCS patients were treated with lumpectomy. Axillary lymph node dissection was performed in 21 patients (84%). All the dissected lymph nodes were free of metastasis. Sentinel lymph node biopsy was performed since 2000.
Radiotherapy was initiated at a median interval of 3.4 weeks after the last surgical procedure (range: 1.4 to 17.7 weeks). The whole breast was irradiated with 6-MV Photons at a median dose of 50.4 Gy (range: 50.0 to 50.4 Gy). One case was treated with a mixed photon-electron beam. Whole breast irradiation was followed by a supplemental boost to the tumor bed for a median total dose of 59.4 Gy (range: 50.4 to 64.4 Gy) to the tumor bed. The tumor bed was boosted with a 6MV-photon or electron beam. Adjuvant hormonal therapy or chemotherapy was not performed regularly.
The follow-up data was not available for one patient, and this patient was excluded from the analysis. Twenty-six cases of 25 patients were finally reviewed. The median follow-up from the initial diagnosis was 67 months (range: 38 to 149 months).
Statistical analysis was done with using the Statistical Package for Social Sciences 12.0 (SPSS Inc., Chicago, IL). The local recurrence free survival rate and the overall survival were estimated by the Kaplan-Meier method. The data was analyzed by the Pearson Chi-Square test for categorical variables. A p-value≤0.05 was considered statistically significant.
RESULTS
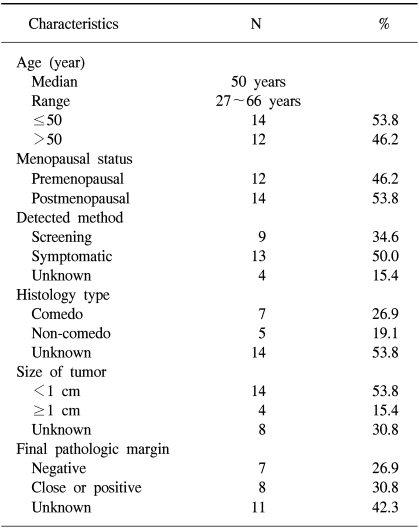
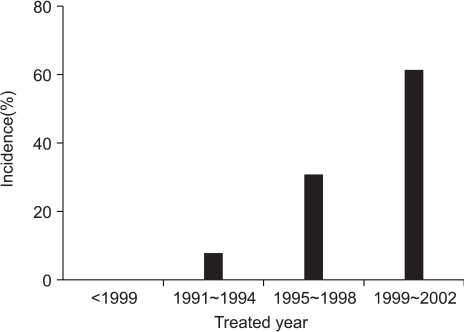
The patients and tumor characteristics are listed in Table 1. Twenty-six cases of 25 patients were included in the analysis. The number of cases treated with BCS-RT for DCIS throughout the study period were 0 (0%) before 1990, 2 (7.7%) from 1991 to 1994, 8 (30.8%) from 1995 to 1998, and 16 (61.5%) from 1999 to 2002 (Fig. 1). The median age at presentation was 50 years (range: 27 to 66 years). Twelve cases (46.2%) were at a premenopausal status. Nine cases of DCIS were (34.6%) were screen-detected and non-symptomatic, and 13 cases of DCIS (50.0%) were symptomatic at presentation. Most of symptomatic cases presented with palpable lumps.
All the cases were managed with BCS. Nine cases (34.6%) underwent lumpectomy and 17 cases (65.4%) underwent partial mastectomy or quadrantectomy. Of the 12 cases whose histology type was known, 7 cases (26.9%) were the comedo type, 1 (3.8%) was the cribriform type, 3 (11.5%) were the papillary type, and 1 (3.8%) was the mixed type. Of 18 cases for whom the pathologic tumor size was known, 14 cases (53.8%) had tumors ≥1 cm in size. The median tumor size was 1.1 cm (range: 0.3 to 4.0 cm). The final pathologic margins were available in 15 cases. Seven cases (26.9%) had negative margins, 8 cases (30.8%) had close or positive margins (30.8%) and 11 cases (42.3%) had unknown margins.
The ipsilateral whole breast was irradiated for all the cases. Most of the cases were given boosts to the tumor bed. The tumor bed was boosted with 6MV-photon in 22 cases (84.6%), or electrons were used in 2 cases (7.7%). Two cases were not boosted. Two-dimensional treatment planning (2D-RTP) was used in 17 cases (65.4%) and 3D-RTP was used in 9 cases (34.6%).
Thirteen patients (52.0%) received adjuvant hormonal therapy and 6 patients (23.1%) received adjuvant chemotherapy.
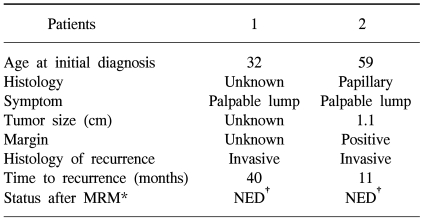
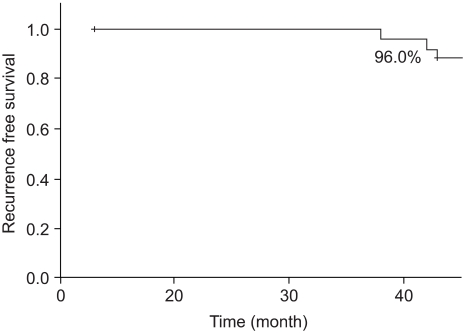
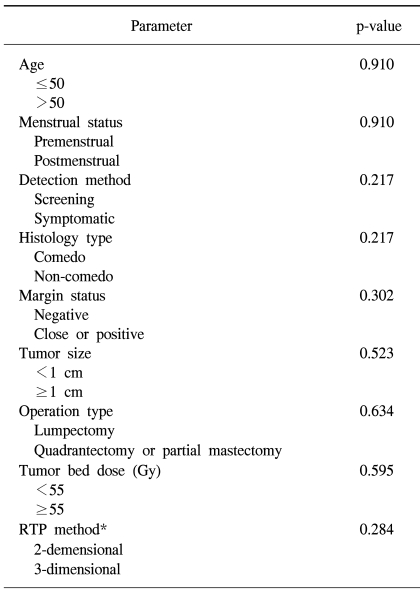
Of all the cases, two cases (7.7%) showed recurrence in the treated breast (Table 2). All the ipsilateral breast tumor recurrence (IBTR) was invasive ductal carcinoma (IDC). All the IBTR was initially detected as palpable lumps. One of the IBTR occurred in a patient who had papillary histology and a positive final margin. At three-year follow up, the local recurrence free survival rate was 96.0% and the overall survival rate was 96.3% (Fig. 2, 3). The median time to local recurrence was 25.5 months. The 2 cases of recurrences developed 11 and 40 months, respectively, after treatment. The risk of lBTR was analyzed, and the clinical factors we examined included patient age at the time of diagnosis, the menopausal status and the method of detection. The pathologic- and treatment-related factors that were analyzed included the operation type, the histology, tumor size, the marginal status, the total radiation dose, the RTP, and hormonal therapy. On the univariate analysis, there were no significant factors associated with IBTR (Table 3).
DISCUSSION
The widespread use of screening mammography has increased the number of patients who are diagnosed with DCIS (1~3,9). This study also showed an increased trend through the years. Since the mid 1980s, radiotherapy has routinely been used as part of BCT for patients suffering with DCIS. The patients treated with BCT after 1990 were enrolled in this study. In Korea, mastectomy was more commonly performed than BCT during the 1980s and early 1990s (7,8,15). Large prospective randomized trials have shown that the use of radiotherapy reduces local recurrences after BCT for DCIS (11~14,16). The National Surgical Adjuvant Breast Project (NSABP) Protocol B-17 (the early report), showed that the 8-year local recurrence rate was 12.1% with performing radiotherapy and this rate increased to 26.8% without radiotherapy (p<0.00005) (11,12). The European Organization for Research and the Treatment of Cancer (EORTC) trial 10,853 showed a 4-year local recurrence rate of 16% for the group treated with local excision alone compared with 9% for the patients treated with local excision plus radiotherapy (p=0.005)(18). Multiple retrospective studies have also shown that excision plus radiotherapy is an effective treatment for DCIS (5,6,17~19). For many DCIS patients, lumpectomy and adjuvant radiotherapy has been a valid treatment option. All patients in our study were BCS-RT. Our study showed a 7.7% rate of IBTR and a 96.0% rate of 3-year local recurrence free survival. All the IBTR was IDC. One case of IBTR was treated with mastectomy.
This study not only lacked information about the hormonal receptors and molecular factors, but it included many young patients, patients with symptomatic lesions and patients with unknown data. Considering that we included patients with these restrictive factors, our result is comparative to the results of the other previous studies.
Many researchers have reported that various factors may be associated with an increased risk for local recurrence when DCIS is treated with BCT (4~6,20~25). Our study analyzed the predictors for IBTR, and we included clinical factors such as age at presentation, the menstrual status and method of detection, the histopathologic factors such as tumor size, final margin status and histology, and the treatment-related factors such as the operation type, the tumor bed dose, the radiation technique and hormonal therapy. By univariate analysis, all the analyzed factors were not correlated with IBTR. Some of the other previous reports were also not able to identify the factors for an increased risk of IBTR (6~8,26).
CONCLUSIONS
Considering that this study included patients with many symptomatic lesions and less detailed data, our results were comparable to other studies. Unfortunately, we did not find the significant factors that would predict local recurrence because of the small number of patients and the short-term follow up period. A longer follow-up period with a larger series of patients is required to evaluate the role of any possible predictive factors and also to confirm our preliminary results.