Neoadjuvant Nivolumab Plus Gemcitabine/Cisplatin Chemotherapy in Muscle-Invasive Urothelial Carcinoma of the Bladder
Article information
Abstract
Purpose
The activity and safety of neoadjuvant nivolumab plus gemcitabine/cisplatin (N+GC) were tested in patients with muscle-invasive bladder urothelial carcinoma (MIBC).
Materials and Methods
In a prospective phase II trial, patients with cT2-T4a N0 MIBC who were eligible for cisplatin and medically appropriate to undergo radical cystectomy (RC) were enrolled. Treatment with nivolumab 3 mg/kg on days 1 and 15 plus GC (cisplatin 70 mg/m2 on day 1, and gemcitabine 1,000 mg/m2 on days 1, 8, and 15) was repeated every 28 days up to 3 or 4 cycles, depending on the surgery schedules. The primary endpoint was pathologic complete response (pCR, ypT0). Secondary endpoints included pathologic downstaging (≤ ypT1), disease-free survival (DFS), and safety.
Results
Between September 2019 and October 2020, 51 patients were enrolled. Neoadjuvant N+GC was well tolerated. Among 49 patients who completed neoadjuvant N+GC, clinical complete response (cCR) was achieved in 59% of intent-to-treat (ITT) population. RC was performed in 34 (69%) patients. pCR was achieved in 24% (12/49) of ITT population and 35% (12/34) of RC patients. Median DFS was not reached. Over a median follow-up of 24 months, 12 patients experienced disease recurrence and were treated with palliative therapy or surgery. Although 12 patients declined surgery and were treated with concurrent chemoradiotherapy, DFS was longer in patients with cCR after neoadjuvant therapy than those without. Preoperative programmed death-ligand 1 (PD-L1) did not correlate with pCR or pathologic downstaging rates.
Conclusion
Neoadjuvant N+GC was feasible and provided meaningful pathologic responses in patients with MIBC, regardless of baseline PD-L1 expression (ONO-4538-X41; CRIS.nih.go.kr, KCT0003804).
Introduction
Cisplatin-based neoadjuvant chemotherapy (NAC) followed by radical cystectomy and bilateral pelvic lymphadenectomy results in substantial tumor downstaging (15%–40% pathologic complete response [pCR]), and has been regarded the standard treatment in patients with muscle-invasive bladder urothelial carcinoma (MIBC) [1,2]. The advantage of NAC is that it facilitates an assessment of the response of the primary cancer to chemotherapy as well as providing an indication as to the likelihood of long-term remission or survival. The disadvantage is that definitive surgery management with a radical cystectomy is potentially delayed for those patients who do not have a major response to the NAC. In addition, it is known that NAC has not been routinely utilized [3], due to possible complications or delays in surgery, a lack of a multidisciplinary approach, or the patients’ refusal. A meta-analysis of data from 11 randomized trials has demonstrated that the absolute survival benefit with NAC is 5% at 5 years [4]. Commonly used cisplatin-based NAC regimens include M-VAC (methotrexate, vinblastine, doxorubicin, and cisplatin), CMV (methotrexate, vinblastine, and cisplatin), and GC (gemcitabine and cisplatin). Patients with MIBC who achieve a pCR (ypT0N0M0 stage) or who are down-staged to non–muscle invasive disease after NAC demonstrate longer overall survival (OS) than those who fail to achieve pCR or are not down-staged [5]. For patients with residual muscle-invasive disease (ypT2-4a) or lymph node–positive disease (pN+) at cystectomy, the median survival is only 3.4 and 2.4 years, respectively [6]. Likewise, disease-free survival (DFS) at 2- or 3-years correlates with 5-year OS of patients undergoing radical cystectomy for MIBC [7].
With the demonstration of significant clinical benefit of immune checkpoint inhibitors (ICIs) in metastatic urothelial carcinoma [8], clinical trials have been developed to evaluate the role of ICIs in neoadjuvant and/or adjuvant settings to improve cure rates and to prevent disease recurrence [9,10]. At the same time, multiple clinical trials are ongoing in metastatic setting to explore the combination of ICIs and platinum-based chemotherapy with the anticipation of synergistic effect and/or reduced risk of developing resistance [11,12]. Nivolumab is a fully-human IgG4 antibody targeting programmed death-1 (PD-1) that was reported to induce durable responses in patients with metastatic urothelial carcinoma who progressed or recurred despite platinum-based chemotherapy [13]. The combination of nivolumab with cisplatin-based chemotherapy demonstrated acceptable safety [14]. Therefore, we designed the present prospective phase II study (ONO-4538-X41) to evaluate the feasibility and activity of the addition of nivolumab to GC NAC for MIBC.
Materials and Methods
In this single-center, prospective, phase II study, eligible patients were aged 20 years or older with clinically-staged T2–T4a N0 MIBC who were medically appropriate to under-go radical cystectomy and eligible for cisplatin by the Galsky criteria [15]. Other inclusion criteria were as follows: an Eastern Cooperative Oncology Group performance status 0 or 1, no prior systemic chemotherapy for MIBC, and and adequate bone marrow (neutrophil count > 1,500/mm3, platelet count > 100,000/mm3), hepatic (aspartate amino-transferase/alanine transaminase ≤ 2.5× upper limit of normal, bilirubin ≤ 1.5 mg/dL), cardiac (left ventricular ejection fraction > 60%) and renal (creatinine clearance ≥ 60 mL/min) functions. Patients with distant and/or lymph node metastases were excluded.
1. Study procedures
All patients underwent transurethral resection of bladder tumor before enrollment to confirm T2–T4a MIBC. Pathologic specimens were examined by a dedicated pathologist (G.Y.K.). Imaging studies with computed tomography (CT) scans or magnetic resonance imaging of the abdomen and pelvis and chest CT were evaluated by a radiologist (C.K.K.) to exclude metastases to lymph nodes and/or distant organs. Eligible patients were reviewed and selected in a multidisciplinary MIBC team meeting comprised of urologic surgeons, a pathologist, a radiologist, a radiation oncologist, and a medical oncologist. NAC with GC (cisplatin 70 mg/m2 on day 1, gemcitabine 1,000 mg/m2 on days 1, 8, and 15) plus nivolumab (3 mg/kg on days 1 and 15) was repeated every 28 days, up to 3 or 4 cycles. Adverse events were recorded and graded in accordance with the National Cancer Institute criteria (Common Terminology Criteria for Adverse Events v4.03). After completion of the planned cycles of nivolumab plus NAC, clinical response was evaluated with CT scans or by the same tests that were initially used to stage the tumor, urine cytology and cystoscopy.
Patients without a progressive disease (PD) after NAC involving nivolumab plus GC were eligible to receive radical cystectomy plus bilateral pelvic lymphadenectomy within 4 to 6 weeks of this determination. If surgery was delayed for any reason, one more cycle of nivolumab plus NAC was allowed. Radical cystectomy included removal of the prostate and seminal vesicles in men and removal of the uterus, vagina and bilateral ovaries in women. The choice of surgical approach (open, laparoscopic, or robot-assisted) was determined by the surgeon’s preference and the patient’s choice. Bilateral pelvic lymphadenectomy was mandatory, and urinary diversions were performed as open procedures. After surgery, pathological stage was evaluated on surgical specimens of primary tumor and lymph nodes. Postoperative, adjuvant chemotherapy was not allowed. All patients were followed every 3 months for 2 years after completion of surgery and every 6 months afterward.
If a patient refused surgery, alternative treatment strategies per our institutional guidelines including bladder preservation chemoradiotherapy was offered [16]. Pre- and post-surgical tumor samples were collected for biomarker analyses. Programmed death-ligand 1 (PD-L1) expression was assessed in formalin-fixed tumor samples using the commercially available PD-L1 immunohistochemistry 22C3 pharmDx assay (Agilent Technologies, Santa Clara, CA). Our pathologist (G.Y.K.) determined the combined positive score (CPS), defined as the total number of tumor cells and immune cells (including lymphocytes and macrophages) stained with PD-L1 divided by the number of all viable tumor cells, then multiplied by 100. A CPS > 1% was classified as PD-L1 positive.
2. Statistical analyses
The primary endpoint of the study was pCR after nivolumab plus GC NAC followed by radical cystectomy in patients with MIBC. A pCR was defined as no evidence of disease both in bladder and regional lymph nodes removed (i.e., ypT0N0) and determined by pathologic report conducted after surgery. Secondary endpoints included safety, clinical complete response (cCR; no visible tumor on imaging studies as well as on urine cytology and cystoscopy) to NAC [16], downstaging rate (i.e., < ypT1N0), DFS, and PD-L1 expression. On the basis of our previous data [17], we hypothesized that a pCR < 20% would suggest lack of activity, and an improvement in the pCR rate to at least 40% was considered to be of clinical interest. We determined that a sample size of 40 patients would provide 90% power to detect the difference at a one-sided significance level of 10%. Assuming a 20% drop-out rate, we planned to recruit at least 48 patients for this study. All patients (intent-to-treat [ITT]) were included in the evaluation of the endpoints. The associations between PD-L1 positivity and clinical outcomes were assessed by the chi-square (pCR) or log-rank (DFS) test. Statistical tests were performed using R packages (http://www.r-project.org).
Results
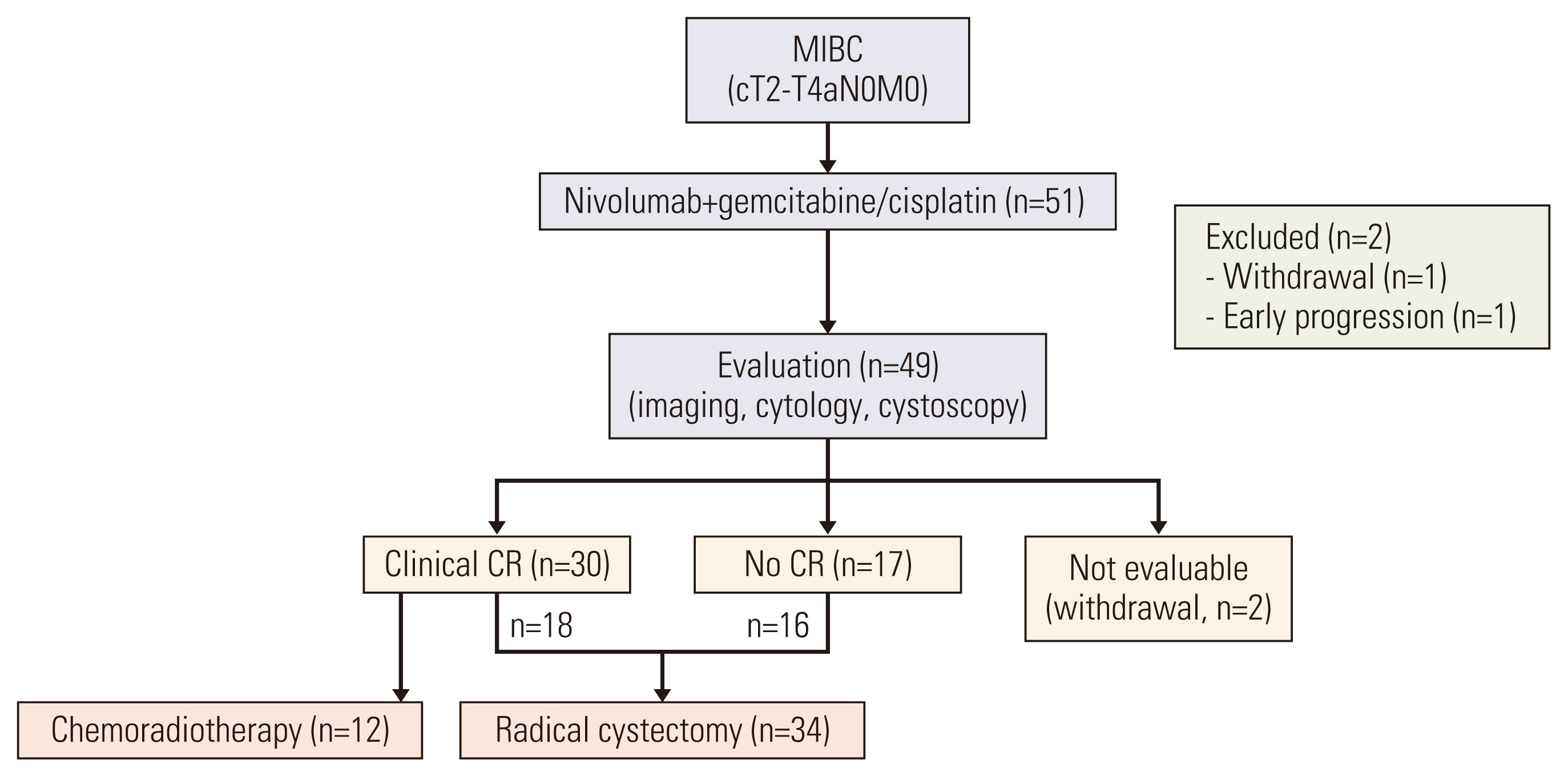
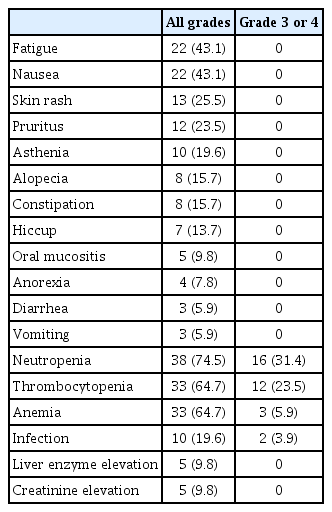
Between August 2019 and October 2020, a total of 51 eligible patients were enrolled and treated with NAC involving nivolumab plus GC (Fig. 1). The patient baseline characteristics are summarized in Table 1. The median age was 66 years (range, 48 to 84 years) and most (84%) were male. Baseline PD-L1 expression status was available in 37 patients. The majority of patients (n=49, 96%) completed the planned cycles of nivolumab plus gemcitabine/cisplatin (median, 3; range 1 to 4) without significant toxicities. The reasons for discontinuing neoadjuvant therapy included early PD (n=1) and withdrawal of consent (n=1). The most commonly observed adverse events included fatigue, nausea and pruritus (Table 2), and no patients discontinued neoadjuvant therapy due to toxicities. Chemotherapy dose reduction was needed in six patients. We observed no chemotherapy-related mortality, or immune-related adverse events except for skin rash and pruritus. After the completion of nivolumab plus NAC, we noted 30 patients with a cCR (59% according to ITT principle; 95% confidence interval [CI], 45% to 72%). In 30 patients with a cCR, 12 refused surgery and were treated with chemoradiotherapy. Before commencing the bladder preservation chemoradiotherapy, our institutional guidelines recommended a maximal transurethral resection [16]. As a result, five of 12 patients who were treated with chemoradiotherapy had pathologic results: ypT0 (n=2), ypTis (n=2), and ypTa (n=1).
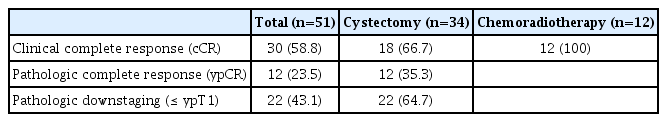
Radical cystectomy was performed in 34 patients, including 18 and 16 patients with and without a cCR, respectively. The median interval between the last dose of NAC and surgery was 36 days (range, 28 to 38 days). Urinary diversions included ileal conduit in 20 patients and neobladder in 14 patients. R0 resection was possible in all surgical candidates, and no patients died within 3 months after surgery due to complications or PD. Pathologic results revealed 12 with pCR (ypT0, 24% according to an ITT principle; 95% CI, 12% to 35%), 4 with ypTis, three with ypTa, three with ypT1, and 12 with ypT2/T3. Pathologic lymph node involvement was found in two patients. If we consider a total of 34 radical cystectomy recipients, the per-protocol pCR rate was calculated to be 35% (Table 3). A strong correlation between cCR and pCR was found (2-sided p=0.037). For example, 13 of 18 patients who achieved cCR and received radical cystectomy had a pCR, whereas only five of 16 patients without cCR undergoing cystectomy had a pCR. At the time of data cutoff (September 2021), the median follow-up duration was 24 months (95% CI, 16 to 26 months). Twelve patients experienced disease recurrence and the sites of recurrence included urinary tract (n=7), lymph nodes (n=3), and distant metastases (n=2). Surgery (transurethral resection, radical cystectomy, or nephroureterectomy) or palliative systemic therapy, depending on the sites of recurrence, was given to those with disease recurrence. Median DFS was not reached (Fig. 2), and the DFS rates at 12- and 24-months were 90% (95% CI, 86% to 94%) and 73% (66%–79%), respectively. For an exploratory purpose, we compared DFS according to the cCR and found a significant difference (hazard ratio, 0.156; 95% CI, 0.043 to 0.571; p=0.005).
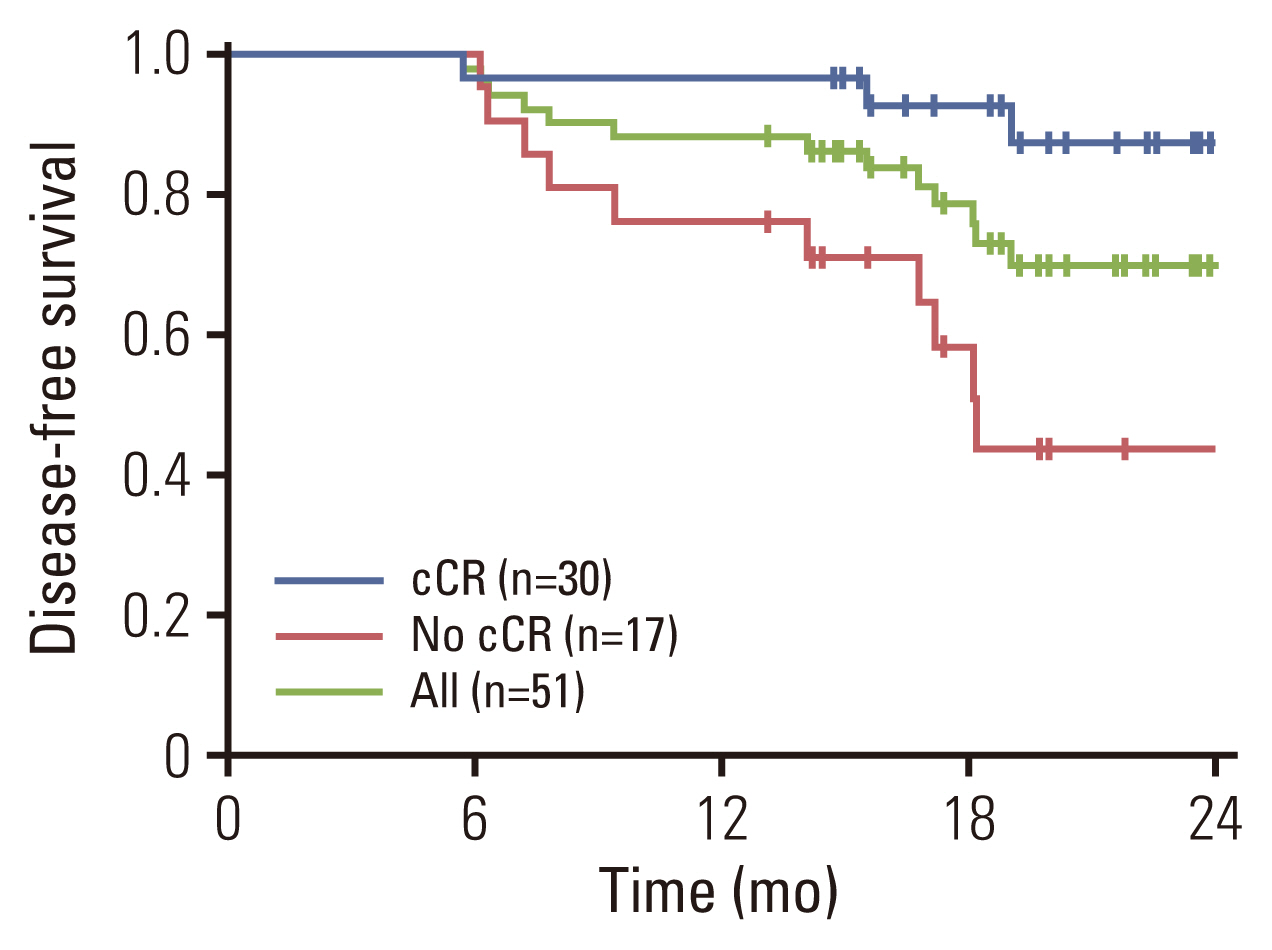
Disease-free survival of all eligible patients (green line, n=51). Blue line denotes survival curve for patients with a clinical complete response (cCR) after neoadjuvant nivolumab plus gemcitabine/cisplatin (n=30). Red line denotes survival curve for those who did not achieve a cCR after neoadjuvant nivolumab plus gemcitabine/cisplatin (n=17).
Overall, 15 of 37 patients (41%) were positive for PD-L1 at baseline. Preoperative PD-L1 (22C3 CPS > 1%) did not correlate with pCR or pathologic downstaging rates: the pCR rate in the PD-L1–positive patients was 30%. After surgery, PD-L1 expression test was available for 21 patients, and only six (29%) had PD-L1–positive tumors. Among 21 patients whose preoperative and postoperative PD-L1 status were both available, the discrepancy rate was calculated to be 38%: positive to negative (n=4) and negative to positive (n=4).
Discussion
We report that the administration of NAC involving nivolumab plus GC was safe in patients with MIBC and did not seem to adversely affect the outcomes of surgery. In line with previous studies involving the combinations of ICIs and cisplatin-based chemotherapy [11,12], the predominant adverse events in this study included mild-to-moderate fatigue, nausea and skin reactions. The present prospective phase 2 study included 51 patients, and 49 completed the planned cycles of neoadjuvant therapy. We observed a 59% cCR and 24% pCR rate according to an ITT principle. Although a significant number of patients (n=12) declined surgery but received bladder preservation chemoradiotherapy, the DFS was significantly longer in patients with a cCR than those without (Fig. 2).
Along with the present phase II study, a number of clinical trials involving neoadjuvant ICIs have published, some of which have included patients who received ICIs alone (PURE-01 [9] ABACUS [10], and NABUCCO [18]), and others of which have included those received ICI plus NAC [19–21]. Despite the small, non-comparative study designs, promising preliminary outcomes with neoadjuvant ICIs will hopefully lead to the inclusion of patients ineligible for cisplatin, in addition to higher pathologic responses with associated survival prolongation. Although nivolumab is already approved for adjuvant therapy for patients with MIBC who are at high risk of recurrence after radical surgery [22], preclinical studies showed that administration of ICIs in preoperative setting could result in greater benefit than postoperative [23]. In syngeneic models of carcinoma with defined antigenicity, neoadjuvant PD-1 immune checkpoint blockade led to a reversal of functional immunodominance among T-cell clones targeting independent antigens and allowed the formation of immunologic memory capable of rejecting tumor cell challenge after surgical resection of tumors [24]. Furthermore, in metastatic setting, although studies so far failed to demonstrate survival benefit [11,12], the addition of ICIs to chemotherapy resulted in higher CR rates than chemotherapy alone. In patients who received NAC, lack of residual disease (i.e., pCR) is one of the most important prognostic factors for patients who underwent radical cystectomy [25,26]. Several clinical studies have tested neoadjuvant ICIs, alone [9,10] or in combination with chemotherapy [18,19], with a reported pCR rate that ranges widely of 31%–46%. Although cross-study comparisons have limitations, and these studies used different drugs for different regimens and different endpoints, it is hoped that ongoing studies will provide answers if the addition of ICIs to NAC would lead to pCR and prolongation of survival.
The present study is limited by a small sample size and the non-comparative design. Only patients with cisplatin-eligible MIBC were able to enter the study. In practice, cisplatin ineligibility is common in this population [27] due to pre-existing medical conditions including renal dysfunction. Another limitation is the higher-than-expected proportion of patients who did not receive radical cystectomy after NAC, which resulted in fewer patients providing pathologic results. Among 30 patients with cCR after nivolumab plus NAC, 12 refused surgery but were treated with chemoradiotherapy. A possible explanation for the drop-out rate is our internal protocol in which MIBC patients with a cCR after NAC could be offered bladder preservation chemoradiotherapy instead of cystectomy [16]. We found the observed DFS was impressive according to the achievement of a cCR after NAC, irrespective of subsequent therapies. Previous studies also suggested that NAC plus nivolumab might achieve durable bladder-intact survival in a subset of patients with MIBC [20], although confirmation in randomized trials is required. Likewise, the pCR rates in ours and others highlight the importance of biomarker-based patient selection. Matched tumor tissue obtained at baseline and at surgery can be evaluated to define biomarkers associated with pCR and resistance to NAC. Unfortunately, the pCR rate did not correlate with PD-L1 positivity in the present study, and we found testing biomarkers after surgery difficult because of the limited amount of tumor tissue available as a result of response to neoadjuvant therapy. Consistent with our findings, BLASST-1 trial involving 41 MIBC patients treated with neoadjuvant nivolumab plus GC reported no correlation of responses with PD-L1 expression [21].
One may ask the role of adjuvant nivolumab in patients who received neoadjuvant ICIs but were at high risk of recurrence after radical cystectomy. In the present study, we had 12 patients with ypT2/T3, but currently no evidence-based treatment options exist for these patients at high risk of recurrence. The use of circulating tumor DNA (ctDNA) has recently emerged as a biomarker for residual disease after surgery [28]. In a prospectively defined retrospective analysis of the IMvigor-010 adjuvant atezolizumab trial [29], patients with MIBC who had ctDNA positive after cystectomy showed poor prognosis, and adjuvant atezolizumab may be associated with improved outcomes compared with observation in patients who are positive for ctDNA and who are at a high risk of recurrence [30].
In conclusion, nivolumab and GC NAC was well tolerated, and this combination may provide an effective neoadjuvant treatment strategy in MIBC, regardless of baseline PD-L1 expression. The findings warrant confirmation in randomized clinical trials.
Notes
Ethical Statement
The study protocol was reviewed and approved by the institutional review board of Samsung Medical Center (Seoul, Korea) (SMC IRB No. 2017-01-006) and conducted in accordance with the ethical principles per the Declaration of Helsinki as well as with the Korean guidance on Good Clinical Practice (KGCP). The study (ONO-4538-X41) was registered in advance (CRIS.nih.go.kr, KCT0003804) and all patients gave written informed consent.
Author Contributions
Conceived and designed the analysis: Jeong BC, Park SH.
Collected the data: Jeong BC, Song W, Sung HH, Hong JY, Park SH.
Contributed data or analysis tools: Kim H, Kwon GY, Kim CK, Park W, Pyo H, Park SH.
Performed the analysis: Kim H, Hong J, Kwon GY, Kim CK, Park W, Pyo H, Park SH.
Wrote the paper: Kim H, Park SH.
Statistical analysis: Hong J.
Drafting the manuscript: Kim H, Park SH.
Critical revision of the manuscript: Kim H, Jeong BC, Hong J, Kwon GY, Kim CK, Park W, Pyo H, Song W, Sung HH, Hong JY, Park SH.
Obtaining funding: Park SH.
Administrative support and supervision: Jeong BC, Park SH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This work was supported by the Samsung Medical Center (Seoul, Korea) Research Fund (OTA1602441, OTA1702441) and in part by Ono Pharma Korea (Seoul, Korea). Study drugs, nivolumab and gemcitabine were kindly provided by Ono Pharma and Dong-A ST (Seoul, Korea), respectively.